Playlist
Show Playlist
Hide Playlist
Pathway Overview and Reactions – Part 2
-
Slides PentosePhosphatePathway Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
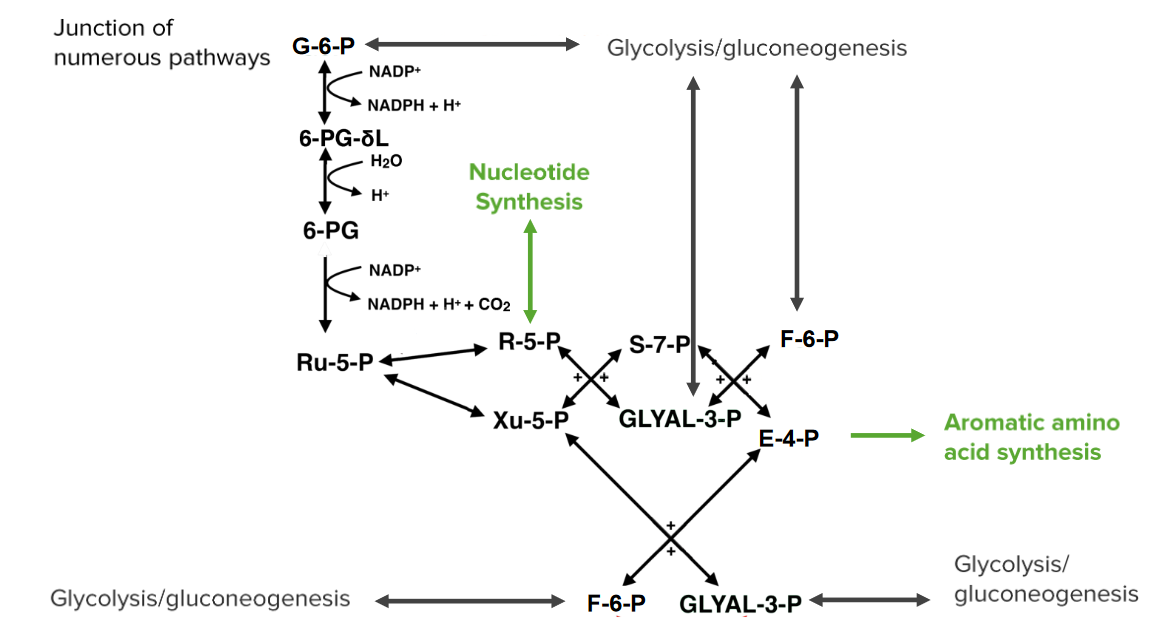
00:02 The alternate pathway for ribulose 5-phosphate is to be converted into xylulose 5-phosphate as you can see here. 00:07 This is a fairly simple reaction, we see ribulose 5-phosphate in the left and xylulose-5-phosphate on the right and the only difference between them is the configuration of one [0:00:15,4] as you can see here. 00:17 The enzyme catalyzing this reaction is known as the ribulose 5-phosphate epimerase and it provides an alternate source of pentose for cells if they should it and also allows for alternative metabolism of xylulose 5-phosphate if cells should encounter it. 00:33 Now, we see the X structure in the middle and the X structure is indicating crossing of the two molecules on the left to make the two molecules to the right of it as we all see. 00:42 So this is a little complicated but I've tried to simplify using some diagrams as we shall see. 00:48 The first of those X2 X structure reactions coverts ribulose-5-phosphate plus the xylulose-5-phosphate to make sedoheptulose 7-phosphate and glyceraldehyde 3-phosphate. 01:01 A lot of words there okay. 01:03 So what’s really happening? What really happens in the process is ribulose 5-phosphate shown here and xylulose 5-phosphate shown here swap parts. 01:12 The parts could swap to make sedoheptulose 7-phosphate and glyceraldehyde 3-phosphate. 01:17 So where the parts come from? Well, the enzymes that catalyzes the reaction is known Transketolase and it has the ability to grab a ketone group of one molecule and move it to another. 01:28 We can see this happening using the co-enzyme thiamine pyrophosphate which actually moves two carbon pieces like I have just described. 01:37 This enzyme is present also and also used in the Calvin cycle as we all see elsewhere. 01:43 Now, here’s ribulose 5-phosphate and all five carbons that it contains. 01:48 Here are two carbons from xylulose 5-phosphate that get transfer onto ribulose 5-phosphate to make sedoheptulose 7-phosphate. 01:58 Now, watch what happens in this process. 02:00 We see first of all, that the top part is xylulose 5-phosphate goes to the top part of the sedoheptulose 7-phosphate And the rest of the ribulose 5-phosphate is left in the bottom. 02:09 So we’ve converted something ahead five carbons ribulose 5-phosphate plus two carbons of xylulose 5-phosphate to make a seven carbon molecule. 02:17 We started with a ketone and we ended up with a ketone which is what sedoheptulose 7-phosphate is. 02:23 If we take the three carbons off of xylulose 5-phosphate we’re left with three carbons to make glyceraldehyde 3-phosphate and that’s what’s there. 02:30 And all the carbons are accounted for. 02:33 Ribulose has five carbons, xylulose has five carbons, sedoheptulose 7-phosphate has seven carbons and glyceraldehyde 3-phosphate has three carbons. 02:43 The next X involves a similar reaction and we’re going to see more swapping of parts. 02:47 But in this reaction, what’s happening is not the moving of the ketone group which involves two carbons but rather the transfer of a single carbon to an aldehyde accepting group. 02:56 Our starting material is sedoheptulose 7-phosphate from before and glyceraldehyde 3-phosphate as you can see here. 03:03 The enzyme catalyzing this reaction is known as transaldolase. 03:07 and the aldo part referring to the aldehyde that the carbon is transferred too. 03:11 So we see here that the products of this reaction or fructose 6-phosphate that has six carbons and a erythrulose 4-phosphate that has four carbons. 03:19 Now this enzyme is deficient and when this enzyme is deficient it leads to liver cirrhosis. 03:26 so it’s a pretty serious deficiency when this enzyme is not present. 03:29 This enzyme is also a target for autoimmunity that happens in multiple sclerosis. 03:34 So it’s a pretty significant health considerations. 03:37 In this reaction, we’re going to see a swapping of parts like we saw in the previous reactions with transketolase but only three carbons removed in the process. 03:46 So here’s the carbon that will move in sedoheptulose 7-phosphate. 03:49 There's a glyceraldehyde 3-phosphate the movement occurs as we see here onto a molecule that becomes erythrulose 4-phosphate. 03:58 We see that the six carbons that are left becomes fructose 6-phosphate and we also see that the glyceraldehyde 3-phosphate itself becomes erythrulose 4-phosphate. 04:09 Now, the next set of reaction is an X that goes downwards. 04:13 And this case we’re going to be making a couple of glycolysis intermediates. 04:16 This reaction involves xylulose 5-phosphate and erythrulose 4-phosphate that makes glyceraldehyde 3-phosphate and fructose 6-phosphate. 04:25 This reaction is also catalyzed by transketolase and you begin to realize that transketolase can handle a variety of different substrates and this are just two more sets of substrates that it can handle. 04:36 In this reaction, we remember the transketolase moves two carbon groups and the movement of two carbons groups requires the co-enzyme thiamine pyrophosphate. 04:45 This enzyme as I said earlier is also involved and used in the Calvin cycle. 04:49 and this reaction we have the movement of the two carbons from xylulose 5-phosphate and leaving behind three carbons to make glyceraldehyde 3-phosphate. 04:57 The two carbons moved as shown here and the three carbons that are left behind become glyceraldehyde 3-phosphate are shown here. 05:03 The other four carbons needed to make the fructose 6-phosphate come from the erythrulose 4-phosphate as you can see here and the reaction is complete. 05:12 Now, this pathway isn’t important so much for the reactions is catalyzing as it is for the connections that are there. 05:18 And the connections that are there are the pathways allows the cell to have enormous flexibility. 05:23 So, I’d like to pose a couple situation that the cell might encounter with this pathway would be valuable. 05:29 One is let’s imagine for the moment that cell has high levels of NADPH within it. 05:34 And the cell is needing to make nucleotides. 05:38 Well, if the cell is needed to make nucleotides and the only way for the cell to make nucleotides will be started to top and move down, that pathway would be block because high NADPH levels would stop both of the reactions that are marked there. 05:51 But there are fortunately other ways for molecules to enter the pentose phosphate pathway and make nucleotides. 05:56 Remember that there were other connections, the glycolysis and gluconeogenesis. 06:00 And so two the intermediates of glycolysis and gluconeogenesis are fructose 6-phosphate and glyceraldehyde 3-phosphate. 06:07 They can enter the pathway as shown in the right side here. 06:10 The glyceraldehyde 3-phosphate can combine with fructose 6-phosphate to make xylulose 5-phosphate and erythrulose 4-phosphate as we’ve already seen. 06:18 The fructose 6-phosphate can also enter from the right side there and combine with erythrulose 4-phosphate to make glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate. 06:27 We move leftwards. 06:28 Those can recombine and make xylulose 5-phosphate and ribulose 5-phosphate. 06:33 Well ribulose 5-phosphate was our target because we need to make nucleotides. 06:37 So we can see using this that the pathway allows entry from a different point than the top that we came in from before. 06:47 Now, let’s imagine we have another situation where we have nucleotide break down going on, we have high levels of NADPH in both cases as before and we need to make aromatic amino acids. 06:58 Is that possible to do? Well, we know that we can't get things in from glucose 6-phosphate using that intermediate because of NADPH levels will prevent it. 07:08 Well, if we have high level of nucleotide breakdown that means that we have an abundance of ribulose 5-phosphate. 07:13 Is it possible then to get from ribulose 5-phosphate to making aromatic amino acids and the answer is of course. 07:19 We follow the red arrows here, we see where the red arrows lead us. 07:22 and we’re going back now and we’ve made xylulose 5-phosphate which combines with ribulose 5-phosphate and we move rightwards, ultimately making erythrulose 4-phosphate for aromatic amino acids synthesis. 07:34 So the answer is yes, we can. 07:36 and we see again more flexibility of the pathway. 07:40 One last scenario I want to pose is that with the cell has very little G6P, glucose 6-phosphate. 07:47 So this might happen with the cell is wanting to make glucose but it doesn’t have enough the intermediate to make it. 07:53 And we have a similar situation of having in abundance of nucleotide because the cell has just broken down a bunch or RNA. 08:00 In that case is going to have a lot ribulose 5-phosphate floating around. 08:04 Well the problem the cell encounter is that the reaction going upwards is essentially irreversible. 08:10 If you remember, the reaction that had the decarboxylation released carbon dioxide and that made the reaction essentially unable to go backwards. 08:18 Well, as the cell able then to make glucose 6-phosphates starting the nucleotide breakdown. 08:23 And the answer is yes. We follow the red arrows as we did before. 08:27 And we see that we can go all the way through the pathway and end up with fructose 6-phosphate and erythrulose 4-phosphate. 08:34 Well, why is that significant? Well, in the process of gluconeogenesis, the precursor of glucose 6-phosphate is fructose 6-phosphate. 08:42 So, the exit point in this case of fructose 6-phosphate allows it to go out of the gluconeogenesis, make glucose 6-phosphate and ultimate to make glucose starting with nucleotides. 08:53 So, this is a really important thing for the cell to be able to do. 08:58 In this lecture, I've talked about it and pentose phosphate pathway some of the intermediates involve and most importantly the ways in which those intermediates connect the other pathways and give the cell tremendous flexibility.
About the Lecture
The lecture Pathway Overview and Reactions – Part 2 by Kevin Ahern, PhD is from the course Carbohydrate Metabolism. It contains the following chapters:
- Pathway Overview and Reactions Part 2
- Pathway Flexibility
Included Quiz Questions
Which statement regarding transketolase is true?
- It requires thiamine pyrophosphate to function.
- It catalyzes the transfer of 1-carbon units.
- Its action is necessary for gluconeogenesis.
- It doesn't require a coenzyme to function.
- Its activity is decreased in vitamin B12 deficiency.
Which statement regarding transaldolase is true?
- It catalyzes the transfer of 3-carbon units.
- It catalyzes the transfer of 2-carbon units.
- It requires thiamine pyrophosphate (TPP).
- It is an enzyme of the oxidative phase of the pentose phosphate pathway.
- It links the pentose phosphate pathway to the citric acid cycle.
Which condition results from a transaldolase enzyme deficiency?
- Liver cirrhosis
- Malaria
- Anemia
- Reactive oxygen species sensitivity
- Acute hemolytic disease
Which compounds are the connectivity points for glycolysis or gluconeogenesis?
- Glyceraldehyde-3-phosphate and fructose-6-phosphate
- Ribose-5-phosphate and glucose-6-phosphate
- Ribulose-5-phosphate and glucose-6-phosphate
- Xylulose-5-phosphate and ribose-5-phosphate
- Sedoheptulose-7-phosphate and erythrulose-4-phosphate.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Very insightful and brilliant explanation. I learned very much in this lecture! School professor just skip much of the importance in PPP!