COVID-19 COVID-19 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). is a respiratory illness caused by severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirus A viral disorder characterized by high fever, dry cough, shortness of breath (dyspnea) or breathing difficulties, and atypical pneumonia. A virus in the genus Coronavirus is the suspected agent. Coronavirus 2 (SARS-CoV-2). First identified in December 2019, COVID-19 COVID-19 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). has had a devastating effect on the world's population, resulting in millions of deaths worldwide and emerging as the most significant global health crisis since the influenza Influenza Influenza viruses are members of the Orthomyxoviridae family and the causative organisms of influenza, a highly contagious febrile respiratory disease. There are 3 primary influenza viruses (A, B, and C) and various subtypes, which are classified based on their virulent surface antigens, hemagglutinin (HA) and neuraminidase (NA). Influenza typically presents with a fever, myalgia, headache, and symptoms of an upper respiratory infection. Influenza Viruses/Influenza pandemic of 1918. The disease is characterized by fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever, cough, and shortness of breath Shortness of breath Dyspnea is the subjective sensation of breathing discomfort. Dyspnea is a normal manifestation of heavy physical or psychological exertion, but also may be caused by underlying conditions (both pulmonary and extrapulmonary). Dyspnea, though symptoms vary widely. Diagnosis is primarily through laboratory testing, with management strategies focusing on supportive care and specific treatments based on disease severity. Vaccination Vaccination Vaccination is the administration of a substance to induce the immune system to develop protection against a disease. Unlike passive immunization, which involves the administration of pre-performed antibodies, active immunization constitutes the administration of a vaccine to stimulate the body to produce its own antibodies. Vaccination remains a key preventive measure.
Last updated: May 7, 2025
COVID-19 COVID-19 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). , or coronavirus disease 2019 Coronavirus disease 2019 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). , is caused by SARS-CoV-2, a novel coronavirus Coronavirus Coronaviruses are a group of related viruses that contain positive-sense, single-stranded RNA. Coronavirus derives its name from “κορώνη korṓnē” in Greek, which translates as “crown,” after the small club-shaped proteins visible as a ring around the viral envelope in electron micrographs. Coronavirus belonging to the family Coronaviridae Coronaviridae Spherical RNA viruses, in the order nidovirales, infecting a wide range of animals including humans. Transmission is by fecal-oral and respiratory routes. Mechanical transmission is also common. There are two genera: coronavirus and torovirus. Coronavirus.
Like all viruses Viruses Minute infectious agents whose genomes are composed of DNA or RNA, but not both. They are characterized by a lack of independent metabolism and the inability to replicate outside living host cells. Virology, SARS-CoV-2 changes over time, with most mutations having little impact on viral properties. However, some changes can affect transmissibility, disease severity, or effectiveness of vaccines and treatments.
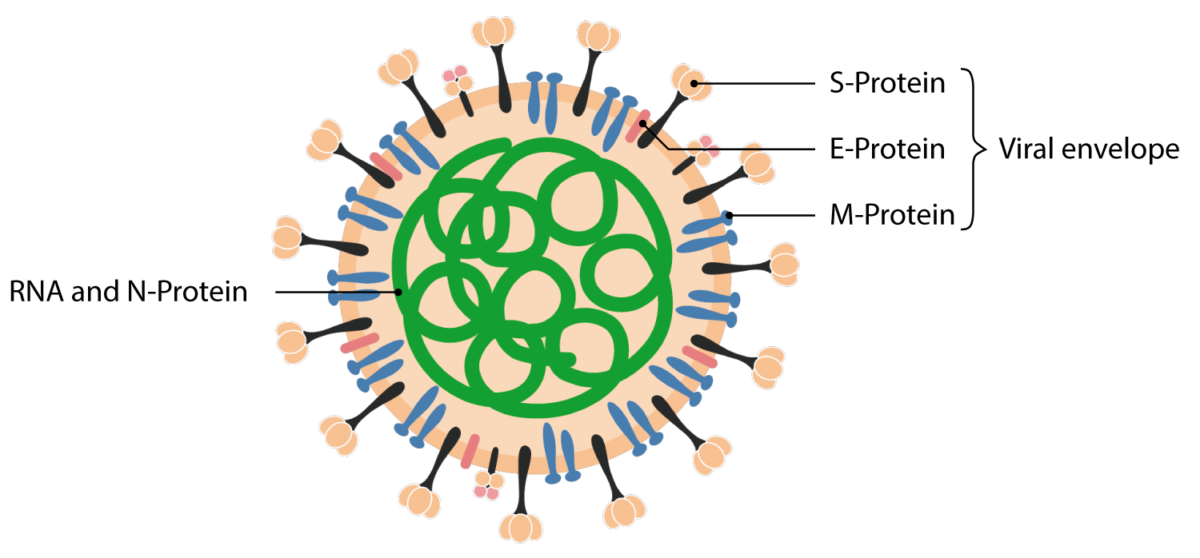
Structural proteins of the SARS-CoV 2 virion.
Image by Lecturio.COVID-19 COVID-19 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). manifests with a range of symptoms from mild to severe, typically appearing 2 days to 2 weeks after exposure.
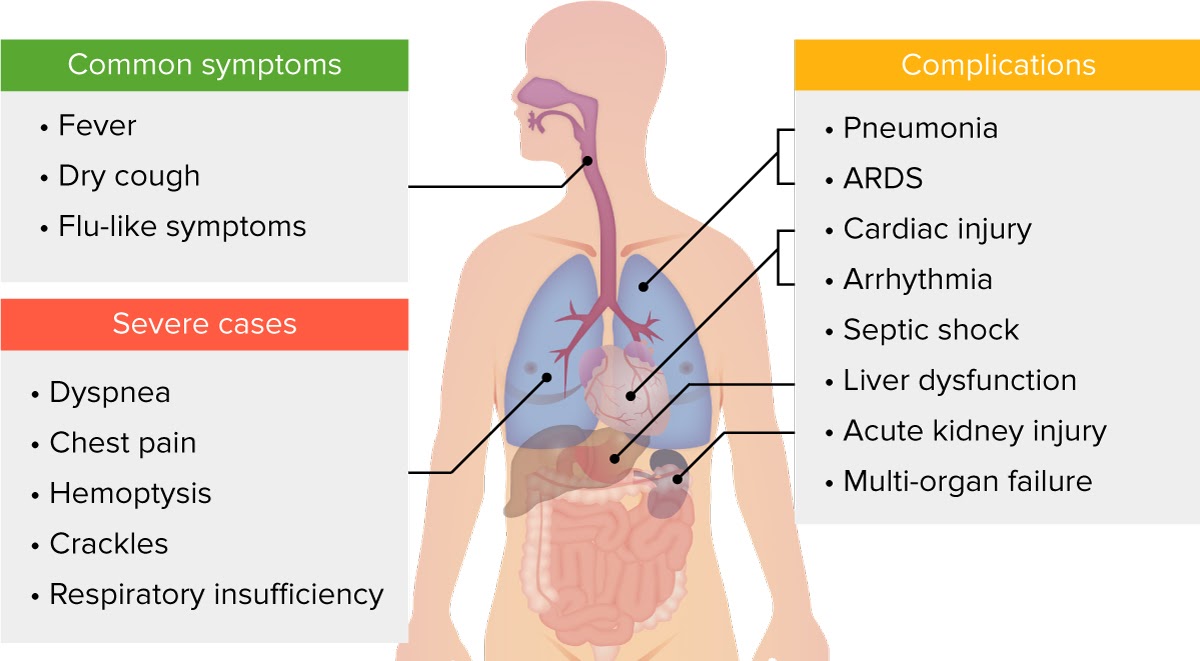
Clinical presentation of COVID-19.
Image by Lecturio.Studies indicate approximately 81% of COVID-19 COVID-19 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). cases are mild, 14% severe, and 5% critical, with higher risk of severe disease in males, older individuals, and those with specific medical conditions.
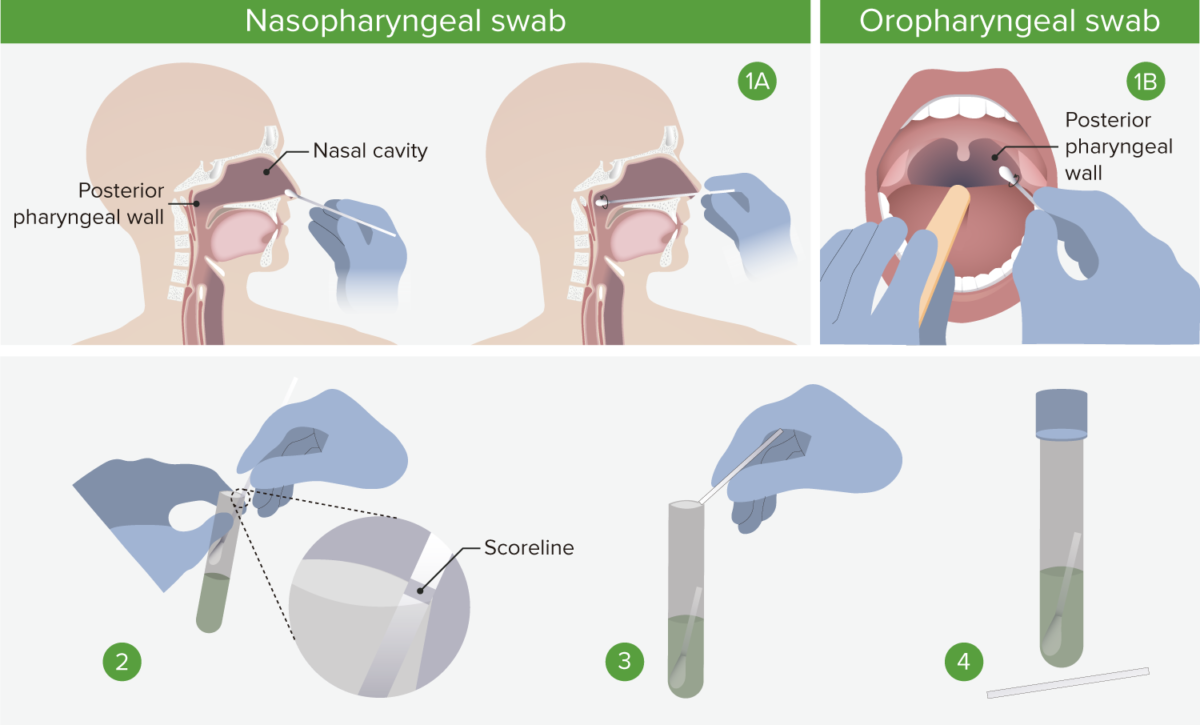
1A. Nasopharyngeal swab: Insert the swab into a nostril parallel to the palate, and carefully slide it forward until a soft resistance is felt. The swab should reach a depth equal to the distance from the nostrils to the outer opening of the ear. Rotate for several seconds to absorb secretions, and then slowly remove. 1B. Oropharyngeal swab: Insert swab into the oral cavity without touching the gums, teeth, or tongue. A tongue depressor may be used. Swab the posterior pharyngeal wall using a rotatory motion. 2. Place swabs immediately into sterile tubes containing 2–3 mL of viral transport media. If both swabs are collected, they should be combined into a single vial. 3. Carefully leverage the swab against the tube rim to break the shaft at the scoreline. 4. Store specimens at 2°C–8°C (35.6°F–46.4°F) for up to 72 hours after collection. If a delay in testing/shipping is expected, store specimens at –70°C (–94°C) or below. Use only synthetic fiber swabs with plastic shafts. Calcium alginate swabs or swabs with wooden shafts may inactivate the virus and inhibit PCR testing.
Image by Lecturio.Vaccination Vaccination Vaccination is the administration of a substance to induce the immune system to develop protection against a disease. Unlike passive immunization, which involves the administration of pre-performed antibodies, active immunization constitutes the administration of a vaccine to stimulate the body to produce its own antibodies. Vaccination is recommended for individuals who are eligible, according to local guidelines.
Multiple vaccines have been developed using different technologies or “platforms” for delivery, with varying efficacies against the initial viral variants and subsequent variants.
| Vaccine Vaccine Suspensions of killed or attenuated microorganisms (bacteria, viruses, fungi, protozoa), antigenic proteins, synthetic constructs, or other bio-molecular derivatives, administered for the prevention, amelioration, or treatment of infectious and other diseases. Vaccination Type | Examples | Technology | Efficacy | Common Side Effects |
|---|---|---|---|---|
| mRNA mRNA RNA sequences that serve as templates for protein synthesis. Bacterial mRNAs are generally primary transcripts in that they do not require post-transcriptional processing. Eukaryotic mRNA is synthesized in the nucleus and must be exported to the cytoplasm for translation. Most eukaryotic mRNAs have a sequence of polyadenylic acid at the 3′ end, referred to as the poly(a) tail. The function of this tail is not known for certain, but it may play a role in the export of mature mRNA from the nucleus as well as in helping stabilize some mRNA molecules by retarding their degradation in the cytoplasm. RNA Types and Structure Vaccines |
|
Non-infective viral mRNA mRNA RNA sequences that serve as templates for protein synthesis. Bacterial mRNAs are generally primary transcripts in that they do not require post-transcriptional processing. Eukaryotic mRNA is synthesized in the nucleus and must be exported to the cytoplasm for translation. Most eukaryotic mRNAs have a sequence of polyadenylic acid at the 3′ end, referred to as the poly(a) tail. The function of this tail is not known for certain, but it may play a role in the export of mature mRNA from the nucleus as well as in helping stabilize some mRNA molecules by retarding their degradation in the cytoplasm. RNA Types and Structure in lipid nanoparticles that code for spike protein | 94-95% (original variants) |
|
| Protein Subunit |
|
Recombinant protein nanoparticle with trimeric spike glycoproteins Glycoproteins Conjugated protein-carbohydrate compounds including mucins, mucoid, and amyloid glycoproteins. Basics of Carbohydrates and adjuvant Adjuvant Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (freund’s adjuvant, bcg, corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. Vaccination | 89% against UK variant (B.1.1.7); 49% against South African variant |
|
| Vector-Based |
|
Replication-incompetent adenovirus Adenovirus Adenovirus (member of the family Adenoviridae) is a nonenveloped, double-stranded DNA virus. Adenovirus is transmitted in a variety of ways, and it can have various presentations based on the site of entry. Presentation can include febrile pharyngitis, conjunctivitis, acute respiratory disease, atypical pneumonia, and gastroenteritis. Adenovirus vector expressing spike protein | AstraZeneca: 62-90% J&J: 66% against moderate-to-severe; 85% against severe disease |
|
| Inactivated Virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology |
|
Inactivated COVID-19 COVID-19 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology with aluminum hydroxide adjuvant Adjuvant Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (freund’s adjuvant, bcg, corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. Vaccination | Variable Variable Variables represent information about something that can change. The design of the measurement scales, or of the methods for obtaining information, will determine the data gathered and the characteristics of that data. As a result, a variable can be qualitative or quantitative, and may be further classified into subgroups. Types of Variables: 50.4% (Brazil), 91.3% (Turkey), 65.3% (Indonesia) |
|
| Respiratory Complications | Cardiovascular Complications | Neurological Complications | Other Complications |
|---|---|---|---|
|
|
|
|
The following conditions are differential diagnoses of COVID-19 COVID-19 Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system but can also cause damage to other body systems (cardiovascular, gastrointestinal, renal, and central nervous systems). :