An essential amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids is an amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids that must come from the diet. Alternatively, a nonessential amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids can be produced by cells and does not require dietary intake. Various substrates undergo a series of processes to make amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids. There are 11 amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids that can be completely synthesized; the other 9 are considered essential and must be included in an individual’s diet. Nonessential amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids are alanine, arginine Arginine An essential amino acid that is physiologically active in the l-form. Urea Cycle, asparagine, aspartic acid, cysteine, glutamic acid Glutamic acid A non-essential amino acid naturally occurring in the l-form. Glutamic acid is the most common excitatory neurotransmitter in the central nervous system. Urea Cycle, glutamine, glycine, proline, serine, and tyrosine. Deficiency in the enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes required for amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids metabolism lead to serious conditions that often present early in life, such as maple syrup urine disease Maple syrup urine disease An autosomal recessive inherited disorder with multiple forms of phenotypic expression, caused by a defect in the oxidative decarboxylation of branched-chain amino acids. These metabolites accumulate in body fluids and render a 'maple syrup' odor. The disease is divided into classic, intermediate, intermittent, and thiamine responsive subtypes. The classic form presents in the first week of life with ketoacidosis, hypoglycemia, emesis, neonatal seizures, and hypertonia. The intermediate and intermittent forms present in childhood or later with acute episodes of ataxia and vomiting. Disorders of Amino Acid Metabolism and phenylketonuria.
Last updated: Dec 13, 2022
Amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids are building blocks for proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis and metabolic intermediates for reactions. Amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids are classified as essential or nonessential. Essential amino acids Essential amino acids Amino acids that are not synthesized by the human body in amounts sufficient to carry out physiological functions. They are obtained from dietary foodstuffs. Basics of Amino Acids must be incorporated into the diet because the body cannot produce them at sufficient levels to meet physiologic demands; nonessential amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids can be produced by the body.
| Biosynthetic family | Amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids | Catabolic product |
|---|---|---|
| α-Ketoglutarate | Glutamate | Glucogenic AA AA Amyloidosis |
| Glutamine | Glucogenic AA AA Amyloidosis | |
| Proline | Glucogenic AA AA Amyloidosis | |
| Arginine Arginine An essential amino acid that is physiologically active in the l-form. Urea Cycle | Glucogenic AA AA Amyloidosis | |
| 3-Phosphoglycerate 3-phosphoglycerate Glycolysis | Serine | Glucogenic AA AA Amyloidosis |
| Glycine | Glucogenic AA AA Amyloidosis | |
| Cysteine | Glucogenic AA AA Amyloidosis | |
| Oxaloacetate Oxaloacetate Derivatives of oxaloacetic acid. Included under this heading are a broad variety of acid forms, salts, esters, and amides that include a 2-keto-1, 4-carboxy aliphatic structure. Citric Acid Cycle | Aspartate | Glucogenic AA AA Amyloidosis |
| Asparagine | Glucogenic AA AA Amyloidosis | |
| Methionine* | Glucogenic AA AA Amyloidosis | |
| Threonine* | Ketogenic or glucogenic AA AA Amyloidosis | |
| Lysine* | Ketogenic AA AA Amyloidosis | |
| Isoleucine* | Ketogenic or glucogenic AA AA Amyloidosis | |
| Pyruvate Pyruvate Derivatives of pyruvic acid, including its salts and esters. Glycolysis | Alanine | Glucogenic AA AA Amyloidosis |
| Valine* | Glucogenic AA AA Amyloidosis | |
| Leucine* | Ketogenic AA AA Amyloidosis | |
| Phosphoenolpyruvate Phosphoenolpyruvate A monocarboxylic acid anion derived from selective deprotonation of the carboxy group of phosphoenolpyruvic acid. It is a metabolic intermediate in glycolysis; gluconeogenesis; and other pathways. Glycolysis & erythrose 4-phosphate | Tryptophan* | Ketogenic or glucogenic AA AA Amyloidosis |
| Phenylalanine* | Ketogenic or glucogenic AA AA Amyloidosis | |
| Tyrosine | Ketogenic or glucogenic AA AA Amyloidosis |
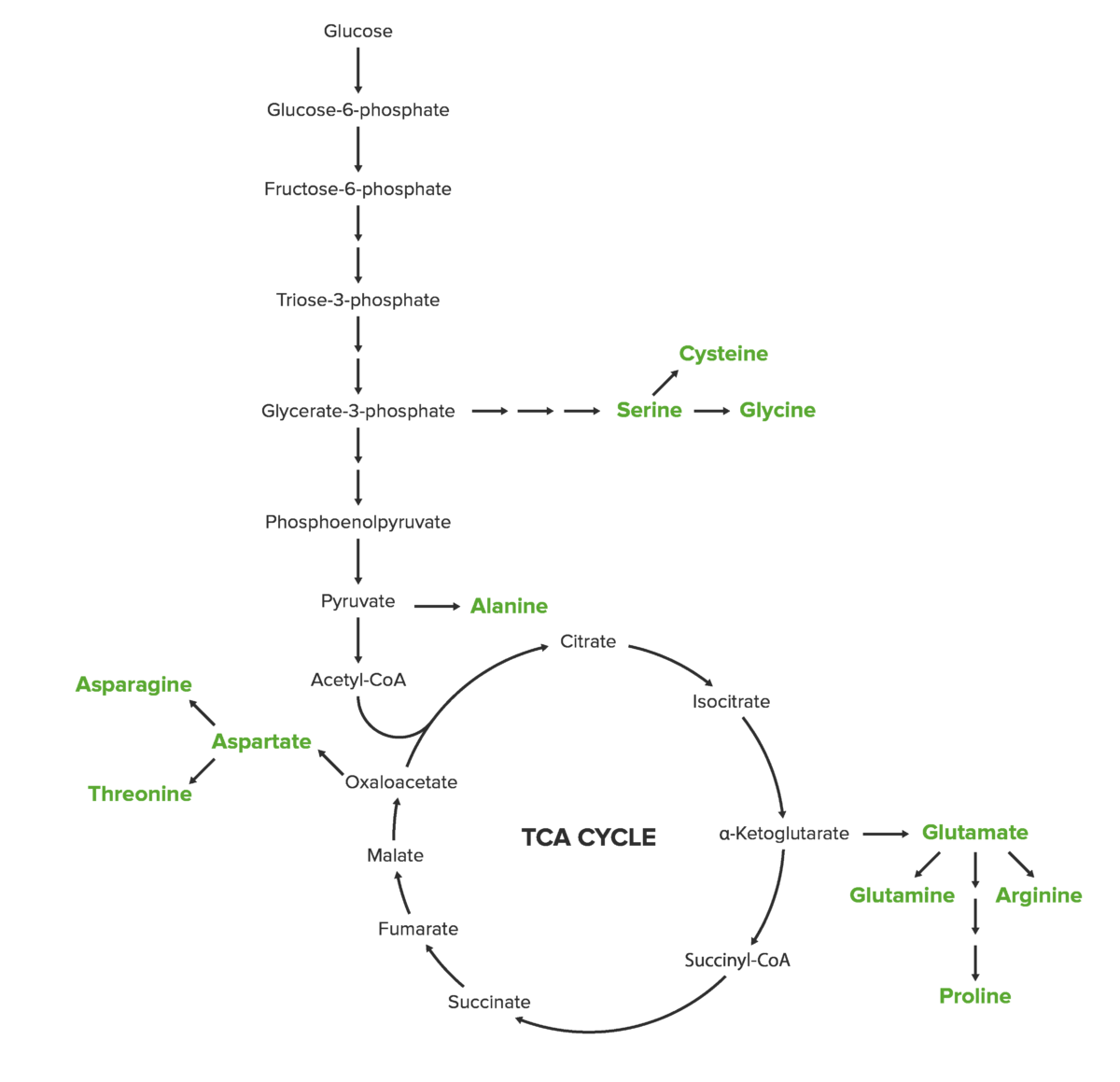
Amino acid biosynthesis overview
CoA: coenzyme A
Glutamate is produced from α-ketoglutarate through the process of amination.
Glutamate serves as the precursor for glutamine.
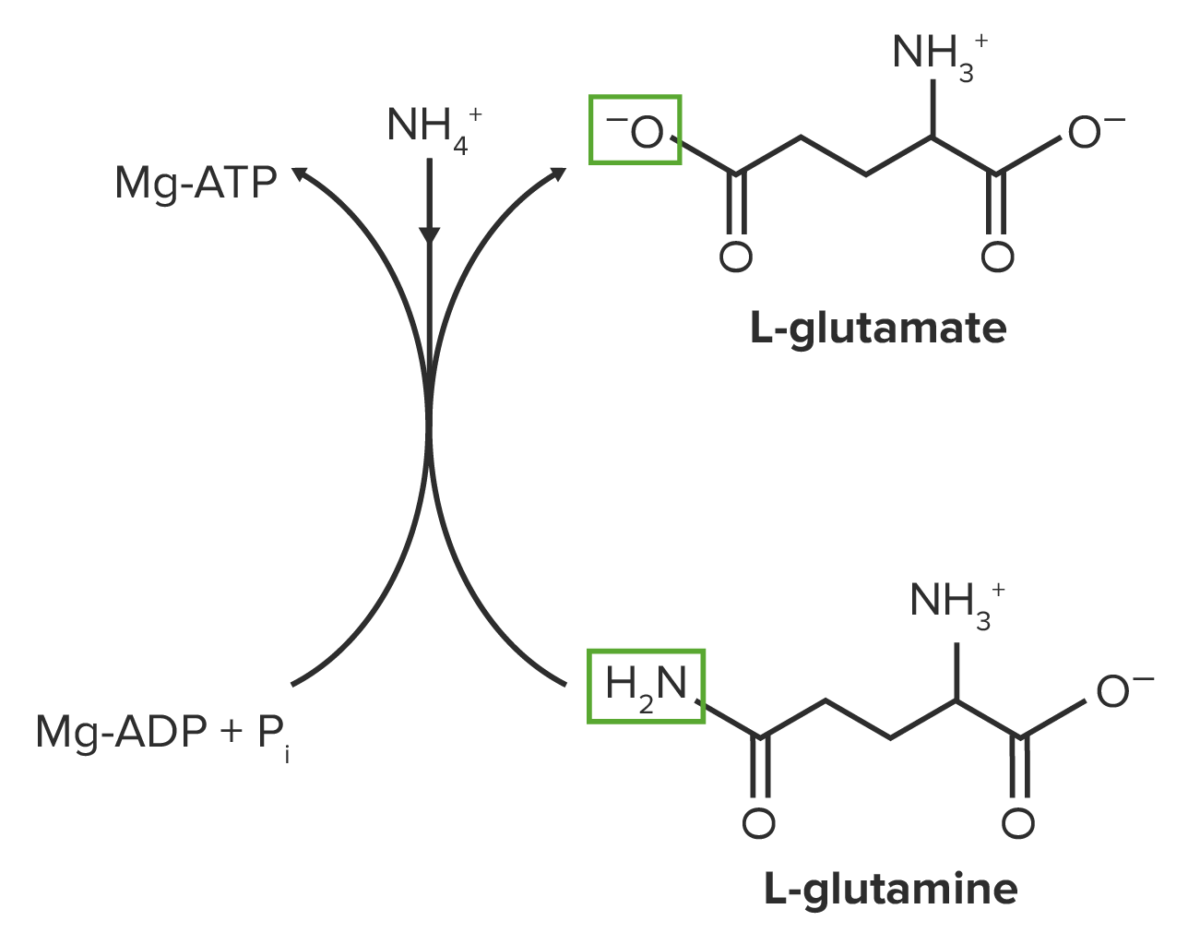
Glutamine synthesis is mediated by glutamine synthetase:
This reaction requires ATP.
Glutamate also serves as the precursor for proline.
3 steps to produce proline from glutamate:
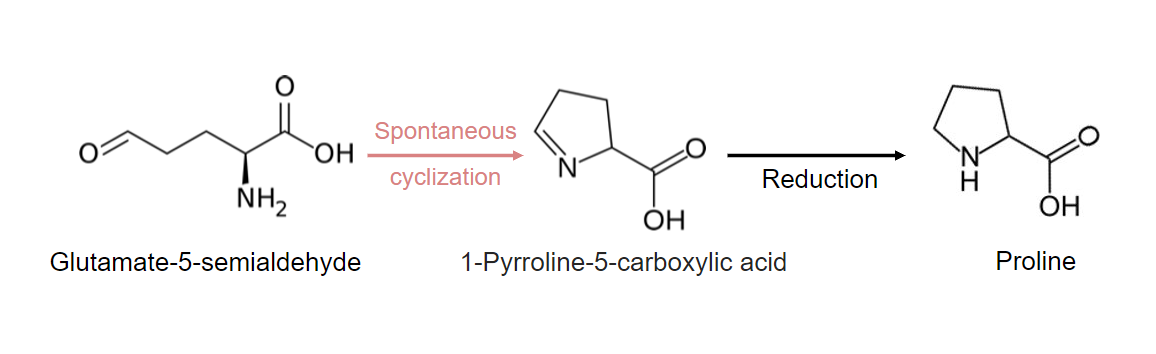
Cyclization and reduction reactions contributing to proline synthesis:
These steps require ATP, NADH, and a cyclization intermediate.
Arginine Arginine An essential amino acid that is physiologically active in the l-form. Urea Cycle is also derived from glutamate.
4 pathways to make arginine Arginine An essential amino acid that is physiologically active in the l-form. Urea Cycle:
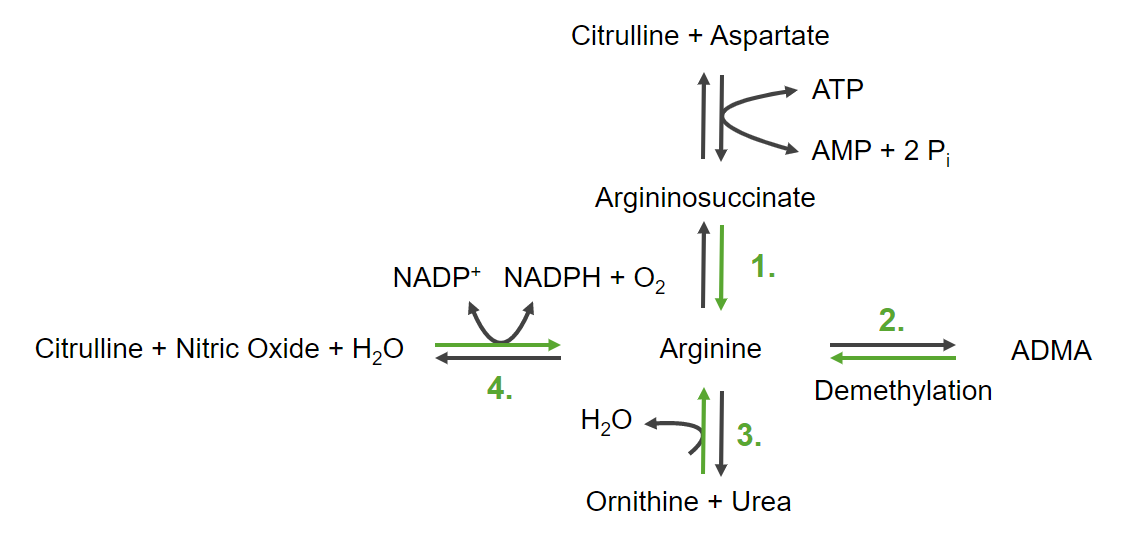
The 4 ways to produce arginine
ADMA: asymmetric dimethylarginine
NADPH and NADP+: nicotinamide adenine dinucleotide
Pi: inorganic phosphate
Serine, glycine, and cysteine are amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids included in the 3-phosphoglycerate 3-phosphoglycerate Glycolysis (3-PG) biosynthetic family. Serine is the first amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids produced in this group, and it subsequently contributes to the production of glycine and cysteine.
Produced by a 3-step pathway:
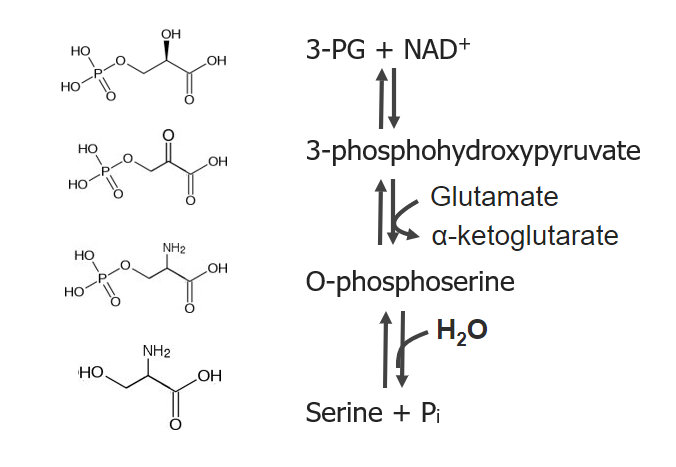
Serine is made via 3 reactions, beginning with 3-phosphoglycerate (PG) and nicotinamide adenine dinucleotide (NAD+)
Image by Lecturio. License: CC BY-NC-SA 4.0Glycine is produced from serine with the removal of a hydroxymethyl group.

Glycine is produced from serine:
This 1-step reaction depends on the removal of a hydroxymethyl group.
Cysteine is produced from serine and homocysteine through a 2-step pathway:
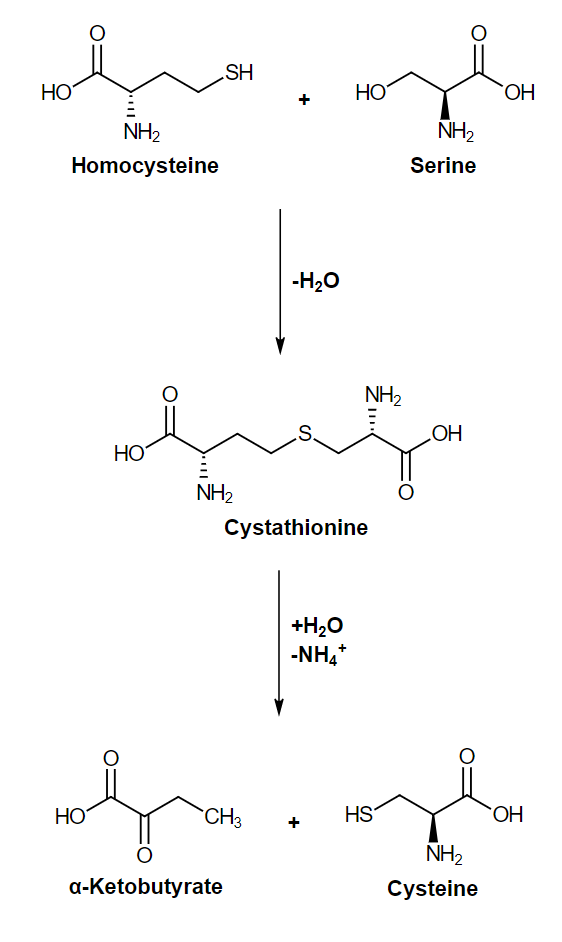
The formation of cysteine from serine and homocysteine
Image: “Cysteine biosynthesis” by David E. License: Public DomainAspartate, lysine, threonine, asparagine, methionine, and isoleucine are amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids that have oxaloacetate Oxaloacetate Derivatives of oxaloacetic acid. Included under this heading are a broad variety of acid forms, salts, esters, and amides that include a 2-keto-1, 4-carboxy aliphatic structure. Citric Acid Cycle as a common precursor.
Aspartate is produced by a transamination Transamination Transamination is the transfer of an amino group from an alpha-AA to an alpha-keto acid, which is an AA with an alpha-keto group (=O) instead of an alpha-amino group (NH2). Catabolism of Amino Acids reaction.
Asparagine is also produced by a transamination Transamination Transamination is the transfer of an amino group from an alpha-AA to an alpha-keto acid, which is an AA with an alpha-keto group (=O) instead of an alpha-amino group (NH2). Catabolism of Amino Acids reaction.
Alanine, valine, and leucine share pyruvate Pyruvate Derivatives of pyruvic acid, including its salts and esters. Glycolysis as a common precursor. Alanine is the only amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids in the pyruvate Pyruvate Derivatives of pyruvic acid, including its salts and esters. Glycolysis biosynthetic family that can be produced by humans.
Alanine is produced through a 2-step reaction.
The aromatic amino acids Aromatic amino acids Amino acids containing an aromatic side chain. Basics of Amino Acids phenylalanine, tryptophan, and tyrosine are included in the phosphoenolpyruvate Phosphoenolpyruvate A monocarboxylic acid anion derived from selective deprotonation of the carboxy group of phosphoenolpyruvic acid. It is a metabolic intermediate in glycolysis; gluconeogenesis; and other pathways. Glycolysis family. Phenylalanine and tryptophan are essential amino acids Essential amino acids Amino acids that are not synthesized by the human body in amounts sufficient to carry out physiological functions. They are obtained from dietary foodstuffs. Basics of Amino Acids; tyrosine is a nonessential amino acid Amino acid Amino acids (AAs) are composed of a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain (R group). Basics of Amino Acids.
Phenylalanine serves as the precursor for tyrosine
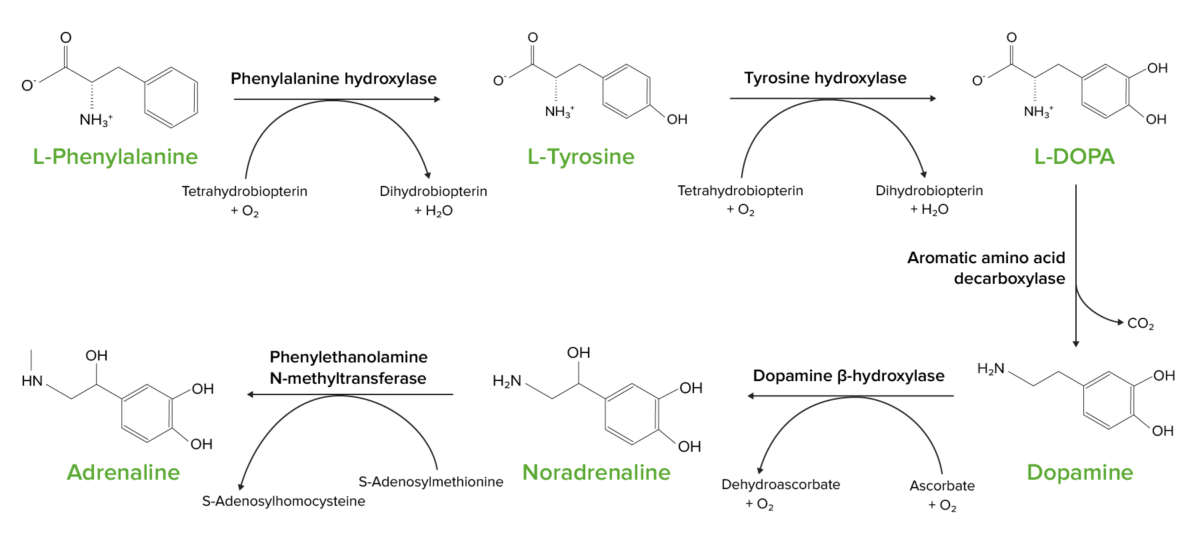
The conversion of L-phenylalanine into L-tyrosine is the 1st step in the upper left.
Tyrosine and phenylalanine are precursors to a number of crucial neurotransmitters and hormones.