Post-translational protein processing (including post-translational modification) is the folding, sorting, cleavage, and modifications required to make a protein functional after it is translated. As the protein folds, it forms complex secondary, tertiary, and quaternary structures. In addition, new functional groups or molecules may be added to the polypeptide chain, including phosphoryl, methyl, or acetyl groups; carbohydrates Carbohydrates A class of organic compounds composed of carbon, hydrogen, and oxygen in a ratio of cn(H2O)n. The largest class of organic compounds, including starch; glycogen; cellulose; polysaccharides; and simple monosaccharides. Basics of Carbohydrates; and lipids Lipids Lipids are a diverse group of hydrophobic organic molecules, which include fats, oils, sterols, and waxes. Fatty Acids and Lipids. Proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis also have to be sorted into the correct intracellular compartment to either carry out their function, be packaged for secretion Secretion Coagulation Studies, or be inserted into the appropriate cellular membrane.
Last updated: Aug 8, 2022
Amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids are the building blocks of proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis. Understanding Understanding Decision-making Capacity and Legal Competence the basics of amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids allows a more comprehensive understanding Understanding Decision-making Capacity and Legal Competence of protein folding Protein folding Processes involved in the formation of tertiary protein structure. Proteins and Peptides and modification.
Amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids that make up proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis are known as α-amino acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance. Each of these amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids has a central carbon known as the “alpha carbon,” which makes 4 bonds:
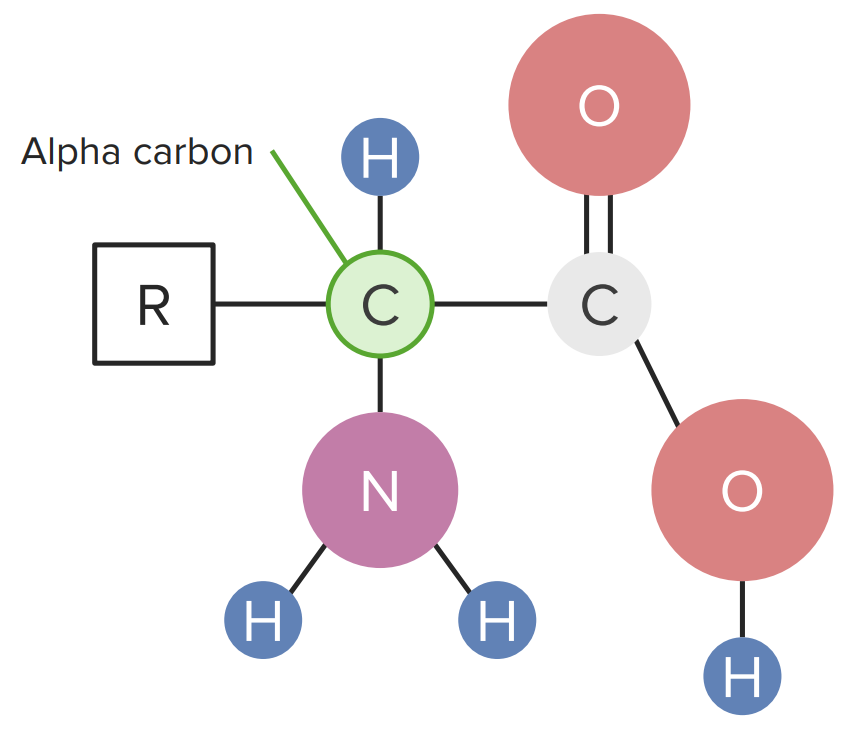
Diagram of an amino acid
Image by Lecturio.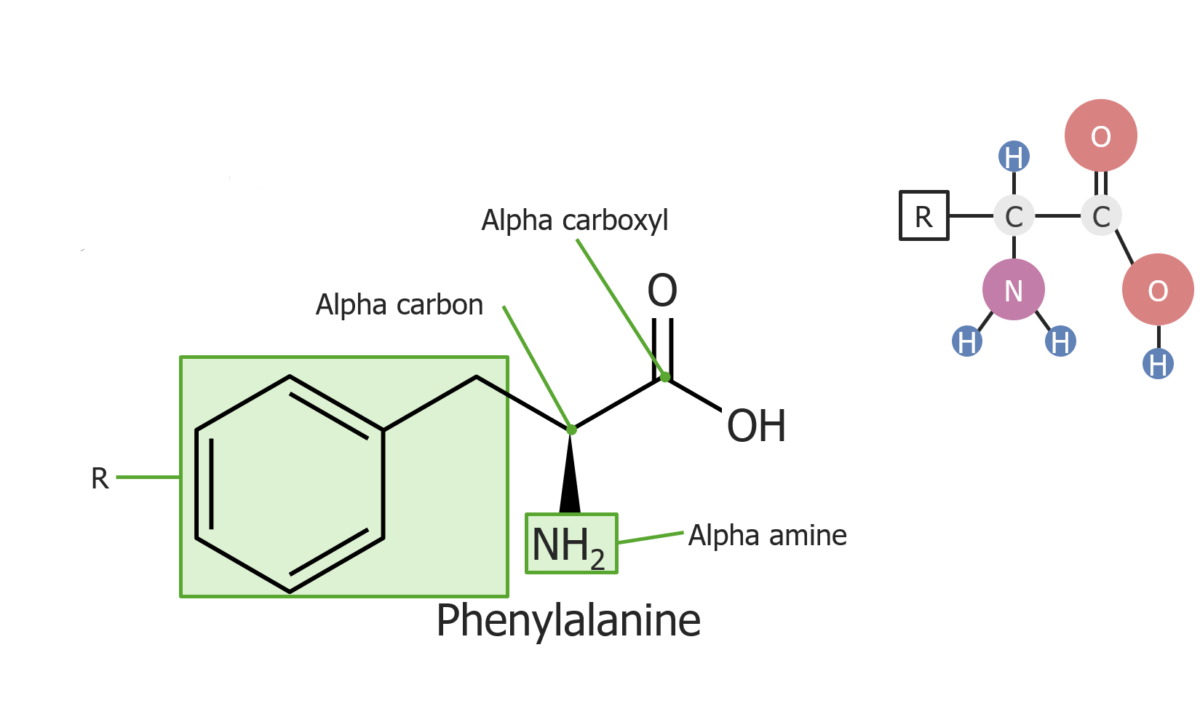
Example of the amino acid phenylalanine
Image by Lecturio.Amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids may be categorized by characteristics of their R groups, which may be:
Protein structure, which is often referred to as protein folding Protein folding Processes involved in the formation of tertiary protein structure. Proteins and Peptides, has 4 levels. These levels are:
Tertiary structure is the complex looping and folding that occurs as a result of interactions and bonding between portions of the protein that are farther apart. Examples of interactions that create tertiary structure include:
In a quaternary structure, multiple subunits of a protein come together to form a single protein.
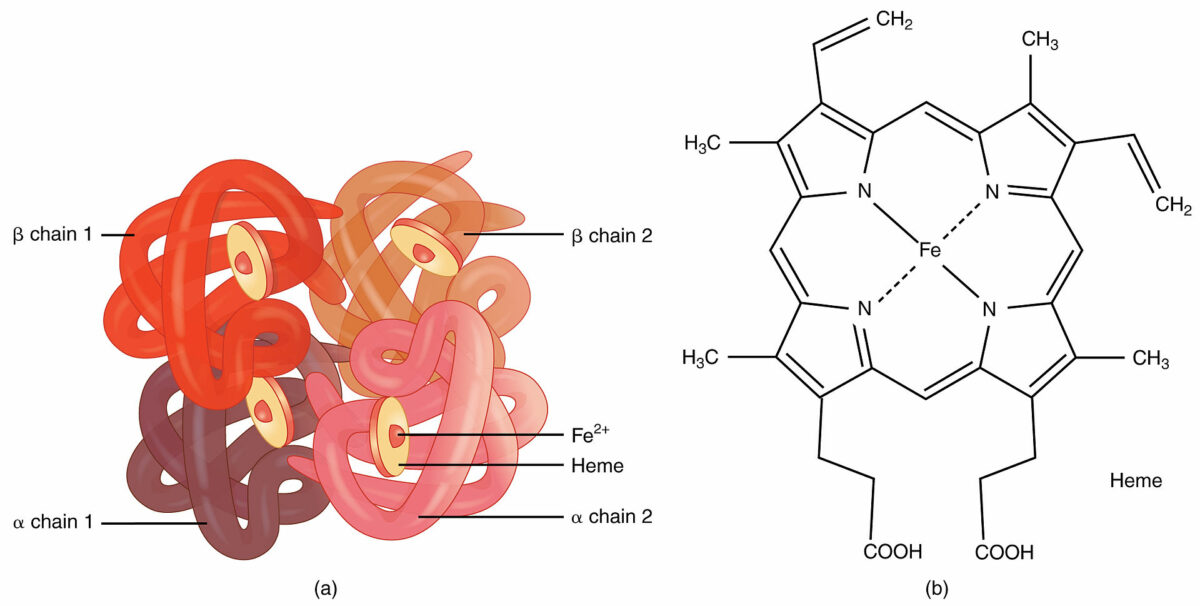
Hemoglobin:
An example of quaternary structure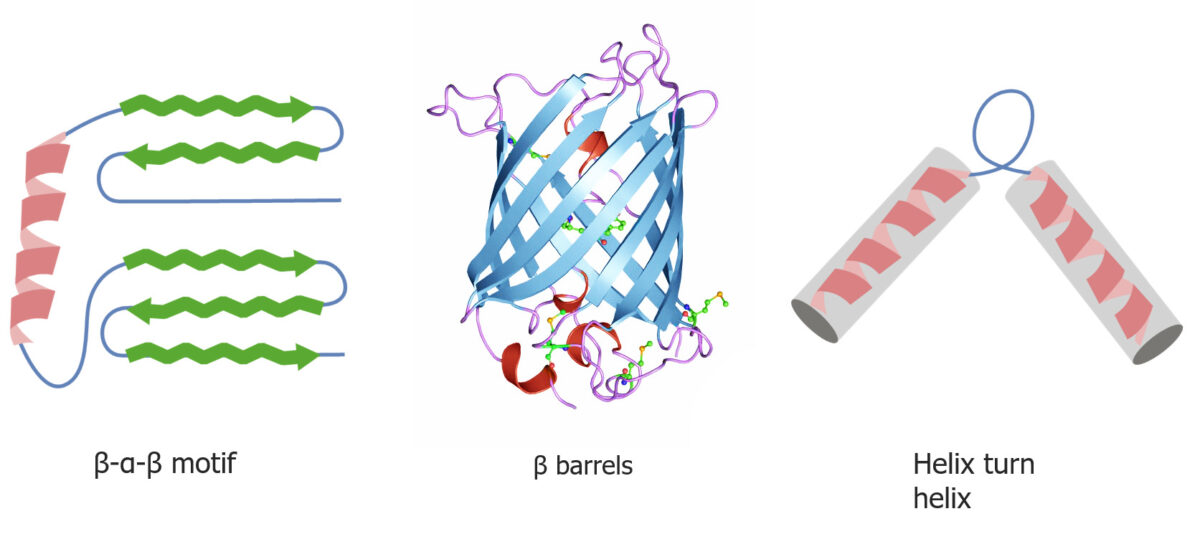
Quaternary and tertiary protein folding motifs
Image by Lecturio.Chaperone proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis assist in protein folding Protein folding Processes involved in the formation of tertiary protein structure. Proteins and Peptides.
A denatured protein is a protein that has been unfolded and is no longer functional. This unfolding occurs under certain conditions, which include changes in:
Proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis need to be sorted and will end up remaining in the cell, being placed on the cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic, or being exported/secreted.
Proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis destined for the cell surface and/or secretion Secretion Coagulation Studies from the cell are synthesized within the rough endoplasmic reticulum Endoplasmic reticulum A system of cisternae in the cytoplasm of many cells. In places the endoplasmic reticulum is continuous with the plasma membrane (cell membrane) or outer membrane of the nuclear envelope. If the outer surfaces of the endoplasmic reticulum membranes are coated with ribosomes, the endoplasmic reticulum is said to be rough-surfaced; otherwise it is said to be smooth-surfaced. The Cell: Organelles (RER):
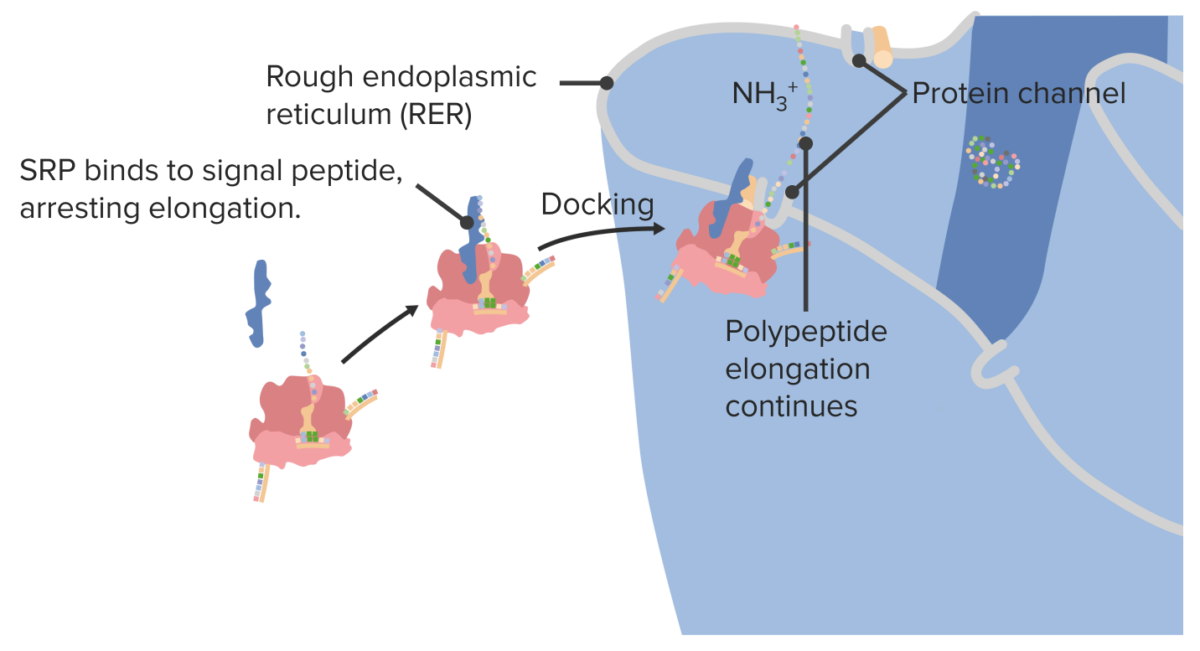
Docking a ribosome on the rough endoplasmic reticulum
SRP: signal recognition protein
After a polypeptide is synthesized, it undergoes further modification in order to form a functional protein. This modification may include cleaving off portions of the polypeptide chain or adding a functional group.
Protein cleavage is the process of removing certain polypeptides in order for the protein to become functional.
Proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis are further modified by the covalent addition of functional groups and other molecules.
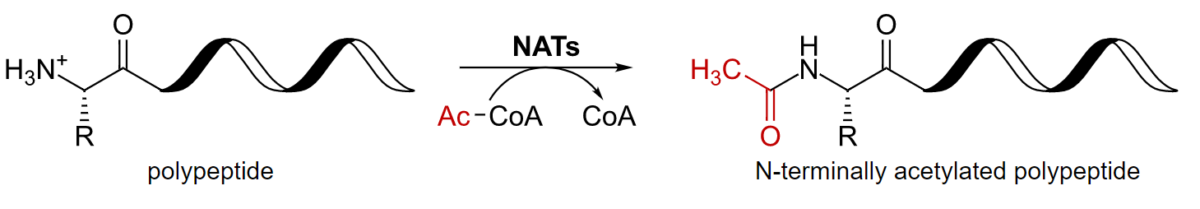
Acetylation of a polypeptide
CoA: coenzyme A
NAT: N-terminal acetyltransferases
Abnormalities in post-translational modification and/or protein folding Protein folding Processes involved in the formation of tertiary protein structure. Proteins and Peptides or sorting can lead to a number of clinically important medical conditions.