What are the types of IV solutions available?
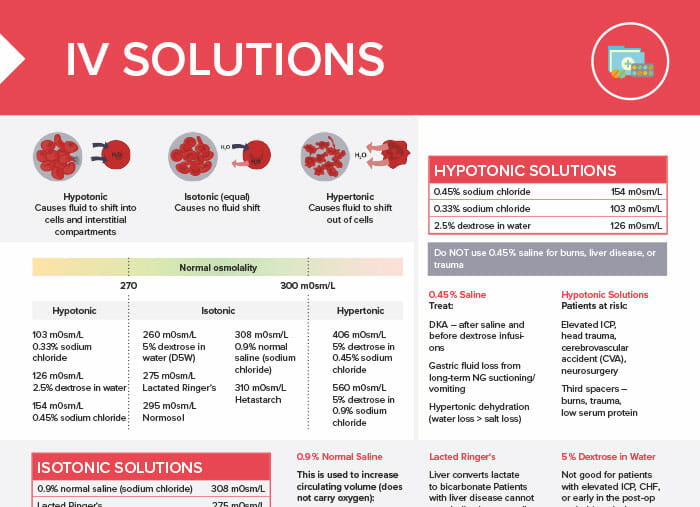
Intravenous (IV) solutions can be broadly categorized into three types based on their osmolality: isotonic, hypotonic, and hypertonic.
The choice of which IV fluid to use depends on the client’s condition, lab values, fluid and electrolyte balance, and acid-base balance. For example, normal saline may be used in a client with hypovolemia, whereas a hypotonic solution might be appropriate for a client with hypernatremia. A hypertonic solution could be used for a client with cerebral edema.
What is osmolality?
Osmolality is a measure of the concentration of solutes in a solution. It’s expressed in osmoles per kilogram of solvent (Osm/kg). In clinical settings, osmolality is commonly used to evaluate the body’s water balance and electrolyte homeostasis.
What are isotonic solutions?
Isotonic solutions have the same osmolality as blood plasma and do not cause any fluid shift. They are used to replace fluids and electrolytes, maintain hydration, and treat hypovolemia.
Examples of isotonic solutions:
| 0.9% normal saline (sodium chloride) | 308 m0sm/L |
| Lactated Ringer solution | 275 m0sm/L |
| 5% dextrose in water (D5W) | 260 m0sm/L |
| Hetastarch | 310 m0sm/L |
| Normosol | 295 m0sm/L |
What are hypotonic solutions?
Hypotonic solutions have a lower osmolality than blood plasma and cause fluid to shift into cells and interstitial compartments. They are used to treat cellular dehydration, reduce edema, and dilute extracellular fluid.
Examples of hypotonic solutions:
| 0.45% sodium chloride | 154 m0sm/L |
| 0.33% sodium chloride | 103 m0sm/L |
| 2.5% dextrose in water | 126 m0sm/L |
What are hypertonic solutions?
Hypertonic solutions have a higher osmolality than blood plasma and cause fluid to shift out of cells. They are used to treat hyponatremia, cerebral edema, stabilize blood pressure, regulate urine output, and minimize the effects of a drastic decrease in serum osmolality.
Examples of hypertonic solutions:
| (D5 ½ NS) 5% dextrose in 0.45% sodium chloride | 406 m0sm/L |
| (D5 NS) 5% dextrose in 0.9% sodium chloride | 560 m0sm/L |
What risks are associated with using hypertonic solutions?
Hypertonic solutions can cause fluid overload, electrolyte imbalances, hyperglycemia, and phlebitis. They should be used with caution in clients with heart failure, renal impairment, or dehydration.
What is a “balanced” or “physiologic” IV solution?
Balanced solutions, like lactated Ringer solution or Plasma-Lyte, closely resemble the composition of plasma and help maintain acid-base balance and electrolyte levels. They are often used when large volumes of fluid are needed, as they can reduce the risk of causing electrolyte imbalances or acidosis compared to saline.
Can all medications be mixed with any IV solution?
No, not all medications are compatible with all IV solutions. Always check medication compatibility with the chosen IV fluid before administration.
Colloids vs crystalloids in IV fluid therapy
- Colloids contain larger molecules (like albumin or starches) and are used to increase the osmotic pressure and draw fluid into the blood vessels, improving intravascular volume.
- Crystalloids (like saline or dextrose) are solutions of small molecules and are used more for hydration or electrolyte balance.