Platelets are small cell fragments involved in hemostasis Hemostasis Hemostasis refers to the innate, stepwise body processes that occur following vessel injury, resulting in clot formation and cessation of bleeding. Hemostasis occurs in 2 phases, namely, primary and secondary. Primary hemostasis involves forming a plug that stops the bleeding temporarily. Secondary hemostasis involves the activation of the coagulation cascade. Hemostasis. Thrombopoiesis takes place primarily in the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis through a series of cell differentiation and is influenced by several cytokines Cytokines Non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, that act as intercellular mediators. They differ from classical hormones in that they are produced by a number of tissue or cell types rather than by specialized glands. They generally act locally in a paracrine or autocrine rather than endocrine manner. Adaptive Immune Response. Platelets are formed after fragmentation Fragmentation Chronic Apophyseal Injury of the megakaryocyte cytoplasm. As a result, platelets have a diameter of 2–3 μm. Nuclei are not present; however, a variety of organelles Organelles A cell is a complex unit that performs several complex functions. An organelle is a specialized subunit within a cell that fulfills a specific role or function. Organelles are enclosed within their own lipid bilayers or are unbound by membranes. The Cell: Organelles are present and aid in different platelet functions.
Last updated: Dec 15, 2025
Platelets are small cell fragments without nuclei, but with a variety of organelles Organelles A cell is a complex unit that performs several complex functions. An organelle is a specialized subunit within a cell that fulfills a specific role or function. Organelles are enclosed within their own lipid bilayers or are unbound by membranes. The Cell: Organelles. Platelets are involved in primary hemostasis Primary hemostasis Hemostasis by adhering to damaged blood vessels and aggregating with one another ( platelet plug Platelet plug Hemostasis).
Description:
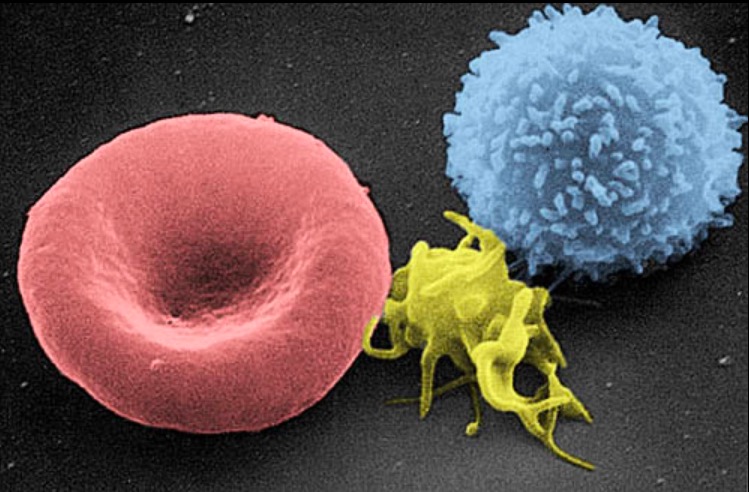
A scanning electron micrograph image of a blood cell:
from left to right, a human red blood cell, a thrombocyte (platelet), and a leukocyte
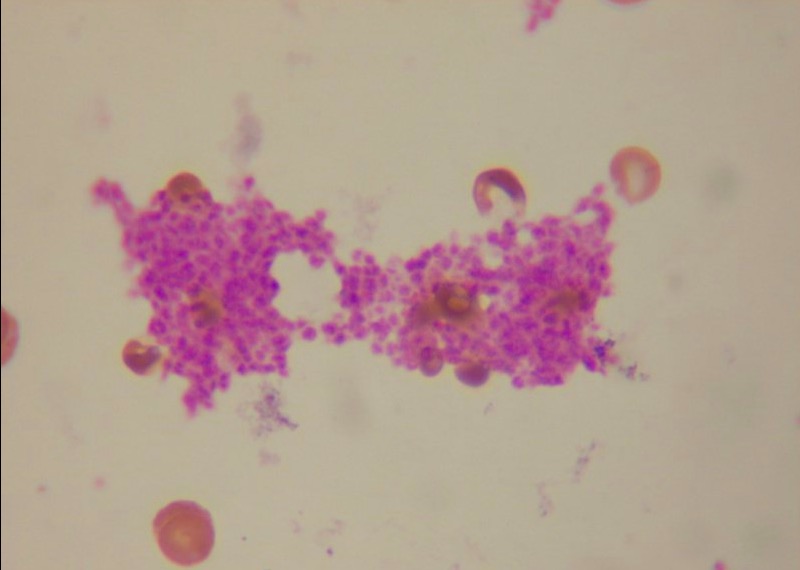
Platelet clumps in a blood smear
Image: “ Platelet clumps in a blood smear” by Tleonardi. License: CC BY 3.0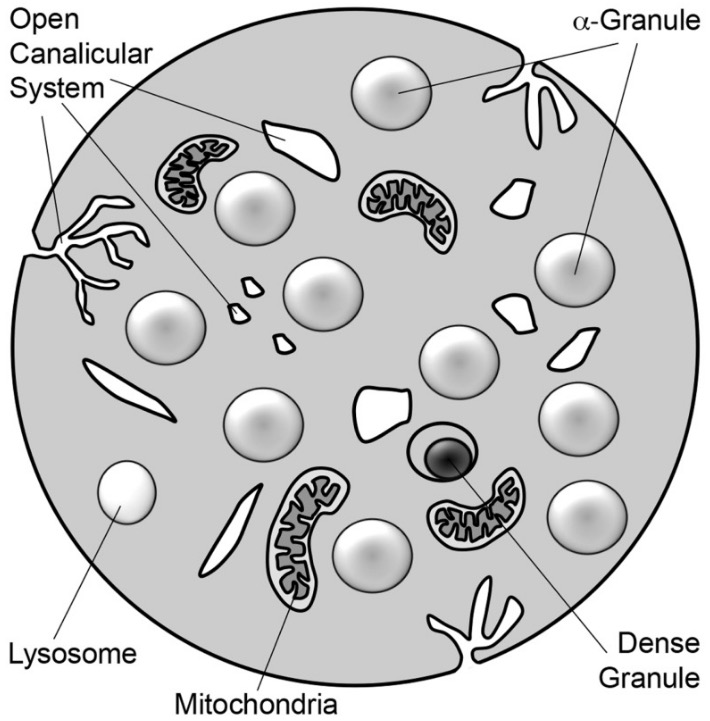
Schematic diagram of a platelet:
The platelet is a 2–3 μm discoid cell containing α-granules, dense granules, lysosomes, and mitochondria. Tunnel invaginations of the plasma membrane form a complex membrane network, the open canalicular system, which courses throughout the platelet interior.
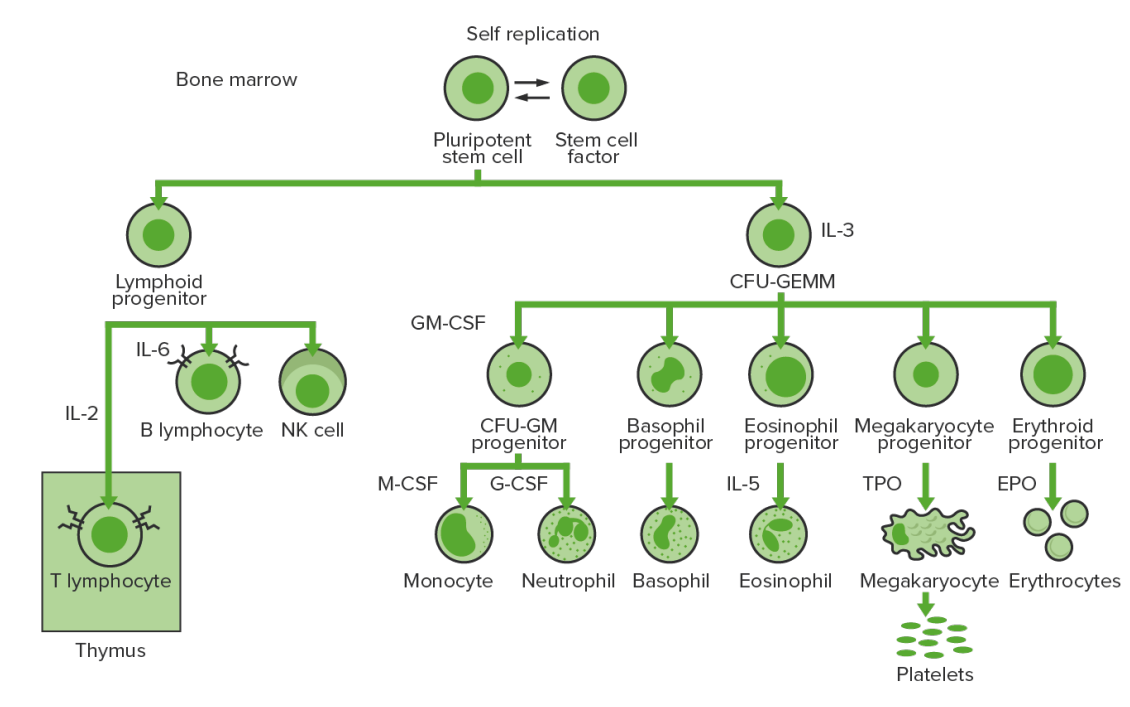
Production and differentiation of the cells in the bone marrow: Hematopoiesis or the production of all blood cells starts with a hematopoietic stem cell, which is prompted to divide and differentiate with appropriate chemical stimuli (hemopoietic growth factors).
CFU-GEMM: colony-forming unit–granulocyte, erythrocyte, monocyte, megakaryocyte
CFU-GM: colony-forming unit–granulocyte-macrophage
GM-CSF: granulocyte-macrophage colony-stimulating factor
M-CSF: macrophage colony-stimulating factor
G-CSF: granulocyte colony-stimulating factor
NK: natural killer
TPO: thrombopoietin
Development takes 1 week on average:
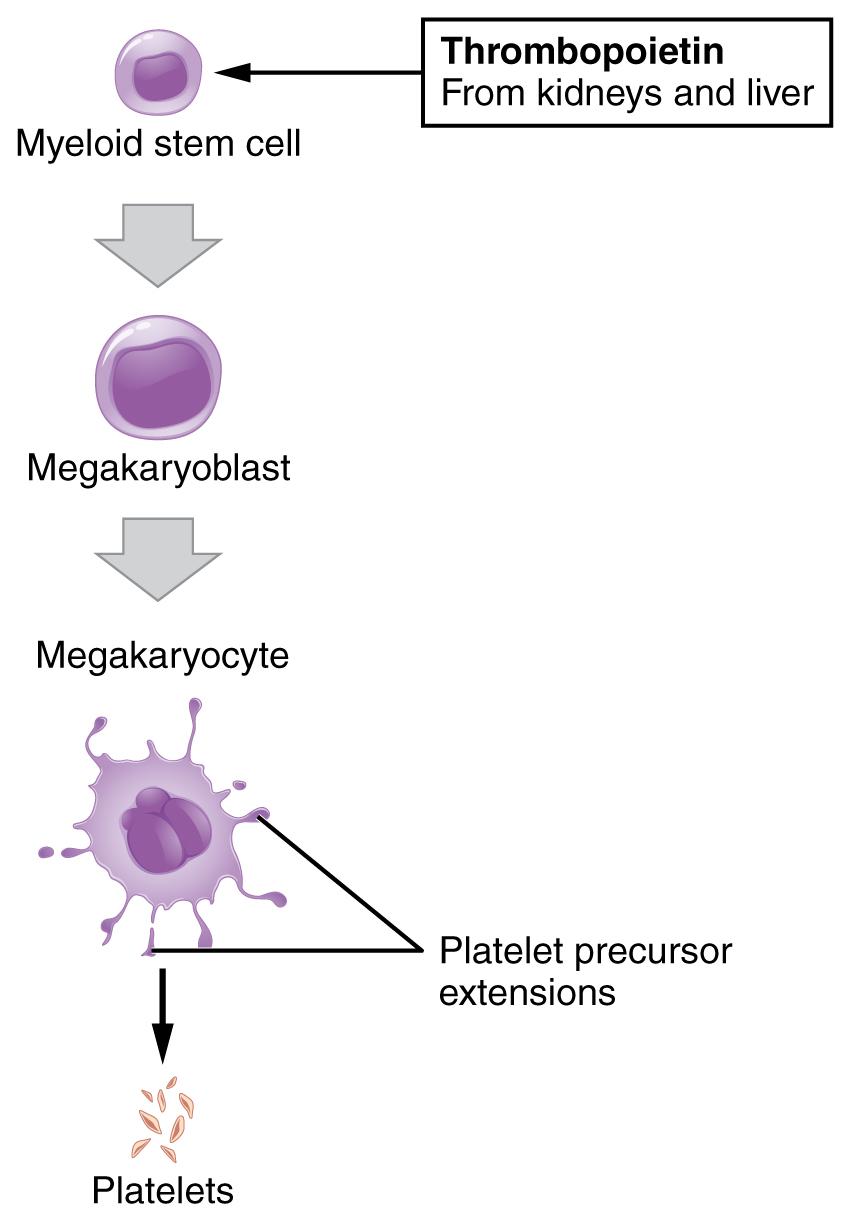
Platelet development:
The hematopoietic stem cells (HSCs) undergo stages to produce common lymphoid and common myeloid progenitors (CMP). Granulocytes, erythrocytes, monocytes, and megakaryocytes arise from CMP, and platelets develop from megakaryocyte fragmentation.
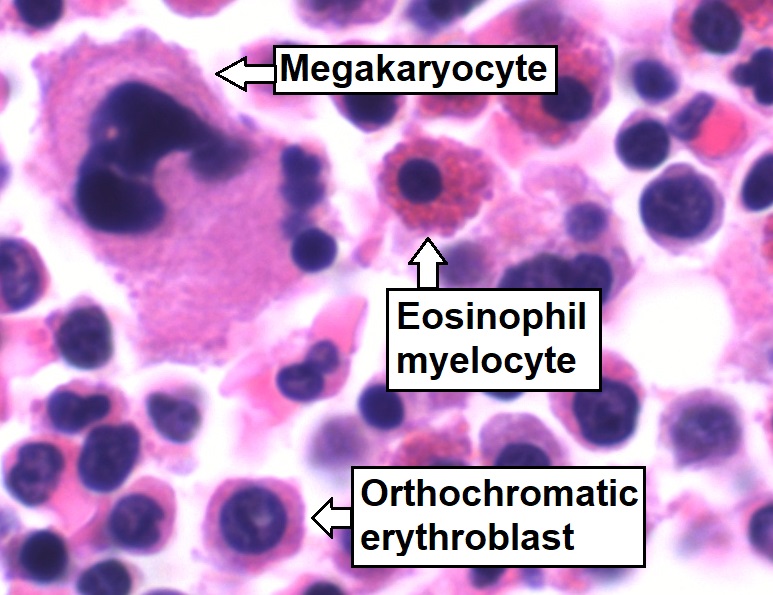
Bone-marrow aspirate showing normal trilineage hematopoiesis: myelomonocytic cells (marked eosinophil myelocyte), erythroid cells (marked orthochromatic erythroblast), and megakaryocytic cells
Image: “Trilineage hematopoiesis” by Mikael Häggström. License: CC0 1.0| Cytokines Cytokines Non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, that act as intercellular mediators. They differ from classical hormones in that they are produced by a number of tissue or cell types rather than by specialized glands. They generally act locally in a paracrine or autocrine rather than endocrine manner. Adaptive Immune Response/growth factors | Activities | Source |
|---|---|---|
| Stem cell factor Stem cell factor A hematopoietic growth factor and the ligand of the cell surface c-kit protein (proto-oncogene proteins c-kit). It is expressed during embryogenesis and is a growth factor for a number of cell types including the mast cells and the melanocytes in addition to the hematopoietic stem cells. Bone Marrow: Composition and Hematopoiesis (SCF) | Stimulates all hematopoietic progenitor cells | Bone-marrow stromal cells |
| Granulocyte-macrophage colony-stimulating factor ( GM-CSF GM-CSF An acidic glycoprotein of mw 23 kda with internal disulfide bonds. The protein is produced in response to a number of inflammatory mediators by mesenchymal cells present in the hemopoietic environment and at peripheral sites of inflammation. GM-CSF is able to stimulate the production of neutrophilic granulocytes, macrophages, and mixed granulocyte-macrophage colonies from bone marrow cells and can stimulate the formation of eosinophil colonies from fetal liver progenitor cells. GM-CSF can also stimulate some functional activities in mature granulocytes and macrophages. White Myeloid Cells: Histology) | Stimulates myeloid progenitor cells Myeloid progenitor cells Stem cells derived from hematopoietic stem cells. Derived from these myeloid progenitor cells are the megakaryocytes; erythroid cells; myeloid cells; and some dendritic cells. Acute Myeloid Leukemia | Endothelial cells, T cells T cells Lymphocytes responsible for cell-mediated immunity. Two types have been identified – cytotoxic (t-lymphocytes, cytotoxic) and helper T-lymphocytes (t-lymphocytes, helper-inducer). They are formed when lymphocytes circulate through the thymus gland and differentiate to thymocytes. When exposed to an antigen, they divide rapidly and produce large numbers of new T cells sensitized to that antigen. T cells: Types and Functions |
| Thrombopoietin (TPO) | Stimulates thrombopoiesis | Kidney, liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy |
| Interleukin-3 (IL-3) | Mitogen for all granulocyte and megakaryocyte-erythrocyte progenitor cells | T helper cells |