Hypercalcemia (serum calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes > 10.5 mg/dL) can result from various conditions, the majority of which are due to hyperparathyroidism Hyperparathyroidism Hyperparathyroidism is a condition associated with elevated blood levels of parathyroid hormone (PTH). Depending on the pathogenesis of this condition, hyperparathyroidism can be defined as primary, secondary or tertiary. Hyperparathyroidism and malignancy Malignancy Hemothorax. Other causes include disorders leading to vitamin D Vitamin D A vitamin that includes both cholecalciferols and ergocalciferols, which have the common effect of preventing or curing rickets in animals. It can also be viewed as a hormone since it can be formed in skin by action of ultraviolet rays upon the precursors, 7-dehydrocholesterol and ergosterol, and acts on vitamin D receptors to regulate calcium in opposition to parathyroid hormone. Fat-soluble Vitamins and their Deficiencies elevation, granulomatous diseases Granulomatous diseases A defect of leukocyte function in which phagocytic cells ingest but fail to digest bacteria, resulting in recurring bacterial infections with granuloma formation. When chronic granulomatous disease is caused by mutations in the cybb gene, the condition is inherited in an X-linked recessive pattern. When chronic granulomatous disease is caused by cyba, ncf1, ncf2, or ncf4 gene mutations, the condition is inherited in an autosomal recessive pattern. Type IV Hypersensitivity Reaction, and the use of certain pharmacological agents. Calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes levels are regulated and affected by factors such as dietary intake and pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance, and the levels of parathyroid Parathyroid The parathyroid glands are 2 pairs of small endocrine glands found in close proximity to the thyroid gland. The superior parathyroid glands are lodged within the parenchyma of the upper poles of the right and left thyroid lobes; the inferior parathyroid glands are close to the inferior tips or poles of the lobes. Parathyroid Glands: Anatomy hormone (PTH), vitamin D Vitamin D A vitamin that includes both cholecalciferols and ergocalciferols, which have the common effect of preventing or curing rickets in animals. It can also be viewed as a hormone since it can be formed in skin by action of ultraviolet rays upon the precursors, 7-dehydrocholesterol and ergosterol, and acts on vitamin D receptors to regulate calcium in opposition to parathyroid hormone. Fat-soluble Vitamins and their Deficiencies, and albumin Albumin Serum albumin from humans. It is an essential carrier of both endogenous substances, such as fatty acids and bilirubin, and of xenobiotics in the blood. Liver Function Tests. Symptoms vary depending on calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes levels and the onset of hypercalcemia. Generally, neuropsychiatric (confusion, altered mental status Altered Mental Status Sepsis in Children), GI ( vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia, abdominal pain Abdominal Pain Acute Abdomen), musculoskeletal ( bone Bone Bone is a compact type of hardened connective tissue composed of bone cells, membranes, an extracellular mineralized matrix, and central bone marrow. The 2 primary types of bone are compact and spongy. Bones: Structure and Types pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways, weakness), and renal ( polyuria Polyuria Urination of a large volume of urine with an increase in urinary frequency, commonly seen in diabetes. Renal Potassium Regulation, polydipsia Polydipsia Excessive thirst manifested by excessive fluid intake. It is characteristic of many diseases such as diabetes mellitus; diabetes insipidus; and nephrogenic diabetes insipidus. The condition may be psychogenic in origin. Arginine Vasopressin Disorders (Diabetes Insipidus)) manifestations are seen. Confirmation of hypercalcemia is required. Correction of the value is based on the albumin Albumin Serum albumin from humans. It is an essential carrier of both endogenous substances, such as fatty acids and bilirubin, and of xenobiotics in the blood. Liver Function Tests levels or after determining the ionized calcium Ionized Calcium Hypocalcemia levels (the metabolically active form), which is followed by determining PTH levels. Subsequent laboratory tests and imaging studies are ordered based on history and presentation. Correction of hypercalcemia depends on its severity. Calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes levels > 14 mg/dL are treated using IV isotonic Isotonic Solutions having the same osmotic pressure as blood serum, or another solution with which they are compared. Renal Sodium and Water Regulation saline hydration, calcitonin Calcitonin A peptide hormone that lowers calcium concentration in the blood. In humans, it is released by thyroid cells and acts to decrease the formation and absorptive activity of osteoclasts. Its role in regulating plasma calcium is much greater in children and in certain diseases than in normal adults. Other Antiresorptive Drugs, and bisphosphonates Bisphosphonates Bisphosphonates are pyrophosphate analogs most well-known for treating osteoporosis by preventing bone loss. Bisphosphonates end in the suffix "-dronate" or "-dronic acid" (e.g., alendronate, risedronate, pamidronate) and bind to hydroxyapatite crystals in bone, inhibiting osteoclast-induced bone resorption. Bisphosphonates. Hemodialysis Hemodialysis Procedures which temporarily or permanently remedy insufficient cleansing of body fluids by the kidneys. Crush Syndrome is considered in rare cases. Treatment of the underlying cause is recommended.
Last updated: May 16, 2024
Calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes is the most abundant mineral in the human body, with 99% found in bone Bone Bone is a compact type of hardened connective tissue composed of bone cells, membranes, an extracellular mineralized matrix, and central bone marrow. The 2 primary types of bone are compact and spongy. Bones: Structure and Types alone. Calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes in blood exists in 3 forms:
Levels:
Importance of calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes:
Bone Bone Bone is a compact type of hardened connective tissue composed of bone cells, membranes, an extracellular mineralized matrix, and central bone marrow. The 2 primary types of bone are compact and spongy. Bones: Structure and Types, intestine, and kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy are involved in homeostasis Homeostasis The processes whereby the internal environment of an organism tends to remain balanced and stable. Cell Injury and Death.
Key elements of calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes regulation:
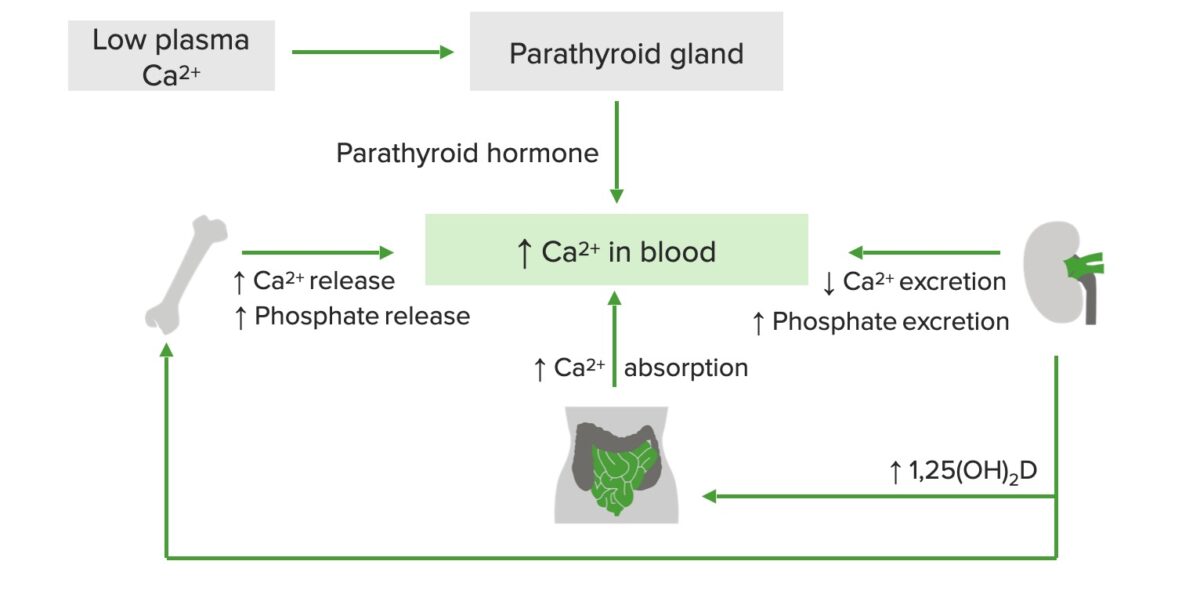
Schematic diagram of calcium regulation:
Low plasma calcium stimulates the release of parathyroid hormone, which increases calcium and phosphate release from the bone, calcium absorption in the GI tract, and vitamin D production in the kidneys. Active vitamin D, in turn, increases calcium release from the bones and calcium absorption in the small intestine.
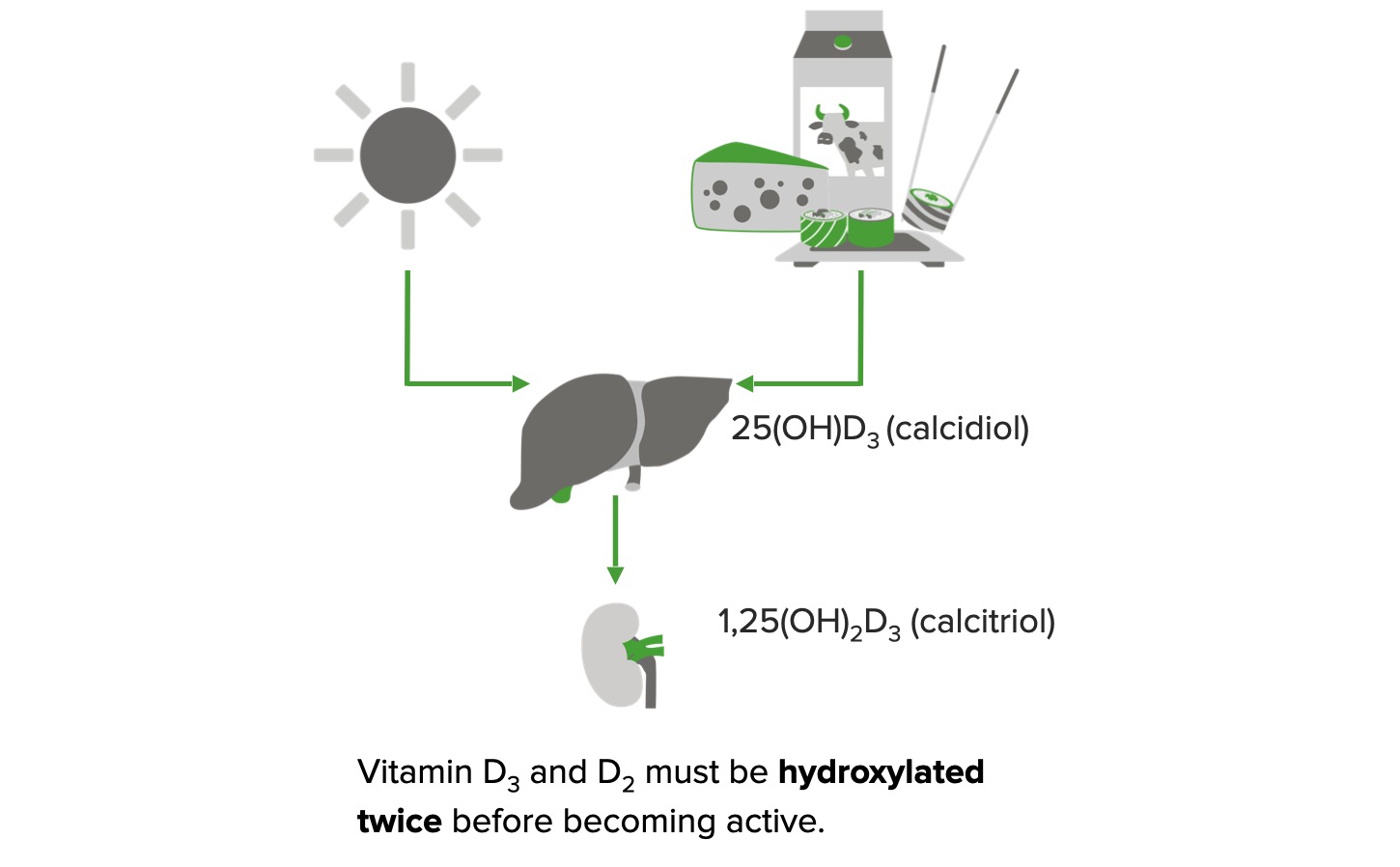
Formation of active vitamin D:
In the presence of sunlight, 7-dehydrocholesterol is converted to cholecalciferol (vitamin D3) in the skin.
Vitamin D2 (plant sources/supplements) and vitamin D3 (animal sources/supplements) are obtained from the diet.
Both forms require a 2-step enzymatic hydroxylation process before they can exert biological effects.
Vitamin D2/D3 is converted to 25-hydroxyvitamin D3 (calcidiol) in the liver. In the kidney, calcidiol is converted to 1,25-dihydroxy vitamin D3 (calcitriol), which is the active form.
Hypercalcemia is characterized by elevated calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes levels and generally results from any 1 of these factors or a combination:
Increased bone resorption Bone resorption Bone loss due to osteoclastic activity. Bones: Remodeling and Healing:
Increased calcium absorption Calcium absorption Digestion and Absorption:
Others:
Manifestations depend on the level and onset of hypercalcemia.
Hypercalcemia with total albumin-corrected calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes < 12 mg/dL:
Hypercalcemia with total albumin-corrected calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes > 12 mg/dL:
Hypercalcemic crisis/severe hypercalcemia:
Mnemonic:
To recall the common clinical symptoms of hypercalcemia, remember “groans, bones, stones, moans, thrones, and psychic overtones”:
| PTH levels | Diagnosis and additional work-up |
|---|---|
| Elevated | Primary hyperparathyroidism Primary hyperparathyroidism A condition of abnormally elevated output of parathyroid hormone due to parathyroid hyperplasia or parathyroid neoplasms. It is characterized by the combination of hypercalcemia, phosphaturia, elevated renal 1, 25-dihydroxyvitamin d3 synthesis, and increased bone resorption. Hyperparathyroidism |
| Normal/slightly increased |
|
| Low |
|