Heme is an iron-containing porphyrin (which is made of 4 pyrrole groups), synthesized mostly in the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis and the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy. Heme is a component of many crucial substances, including cytochromes, myoglobin Myoglobin A conjugated protein which is the oxygen-transporting pigment of muscle. It is made up of one globin polypeptide chain and one heme group. Rhabdomyolysis, and hemoglobin. Biologic functions include the transportation of gases (e.g., O2), and electron transfer. Biosynthesis Biosynthesis The biosynthesis of peptides and proteins on ribosomes, directed by messenger RNA, via transfer RNA that is charged with standard proteinogenic amino acids. Virology of heme is an 8-step process initiated by the synthesis Synthesis Polymerase Chain Reaction (PCR) of aminolevulinic acid. Iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements availability affects heme production, as the last step involves insertion of ferrous ion. Iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements is obtained from the diet and from the breakdown of heme-containing products. In the process of catabolism, heme is converted into bile Bile An emulsifying agent produced in the liver and secreted into the duodenum. Its composition includes bile acids and salts; cholesterol; and electrolytes. It aids digestion of fats in the duodenum. Gallbladder and Biliary Tract: Anatomy pigments, out of which bilirubin is excreted. Mutations involving the enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes in heme synthesis Synthesis Polymerase Chain Reaction (PCR) lead to a group of disorders known as porphyrias Porphyrias Porphyrias are a group of metabolic disorders caused by a disturbance in the synthesis of heme. In most cases, porphyria is caused by a hereditary enzyme defect. The disease patterns differ depending on the affected enzyme, and the variants of porphyria can be clinically differentiated between acute and nonacute forms. Porphyrias, and a defect in the catabolism of heme causes hyperbilirubinemias.
Last updated: Apr 25, 2025
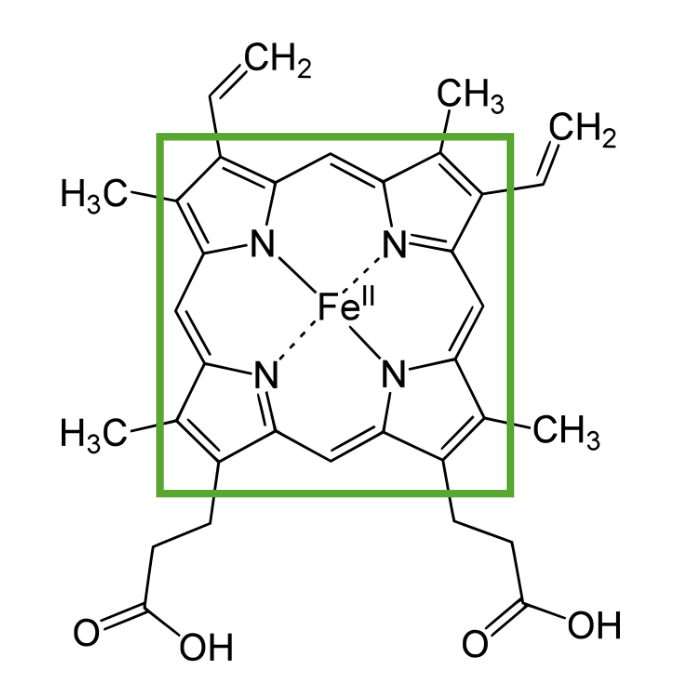
Structure of heme showing a porphyrin ring with a ferrous item in the center
Image by Lecturio.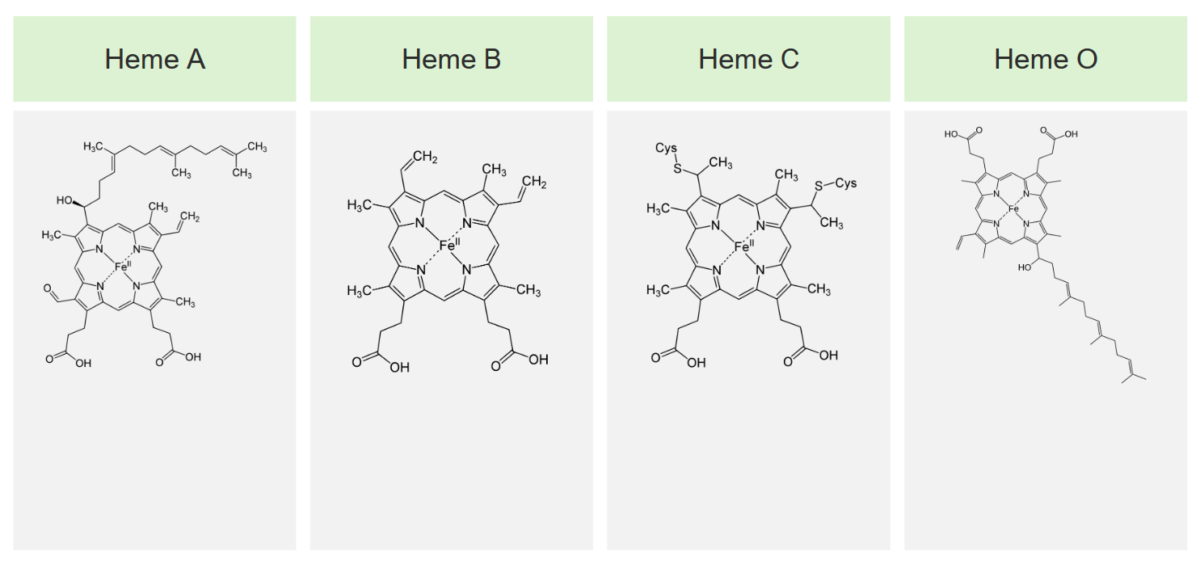
The 4 main types of heme and their structures
Image by Lecturio.Heme is synthesized in the normoblasts, but not in the mature erythrocytes Erythrocytes Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology. The biosynthesis Biosynthesis The biosynthesis of peptides and proteins on ribosomes, directed by messenger RNA, via transfer RNA that is charged with standard proteinogenic amino acids. Virology of heme takes place in 8 steps.
Step 1 is the synthesis Synthesis Polymerase Chain Reaction (PCR) of aminolevulinic acid.
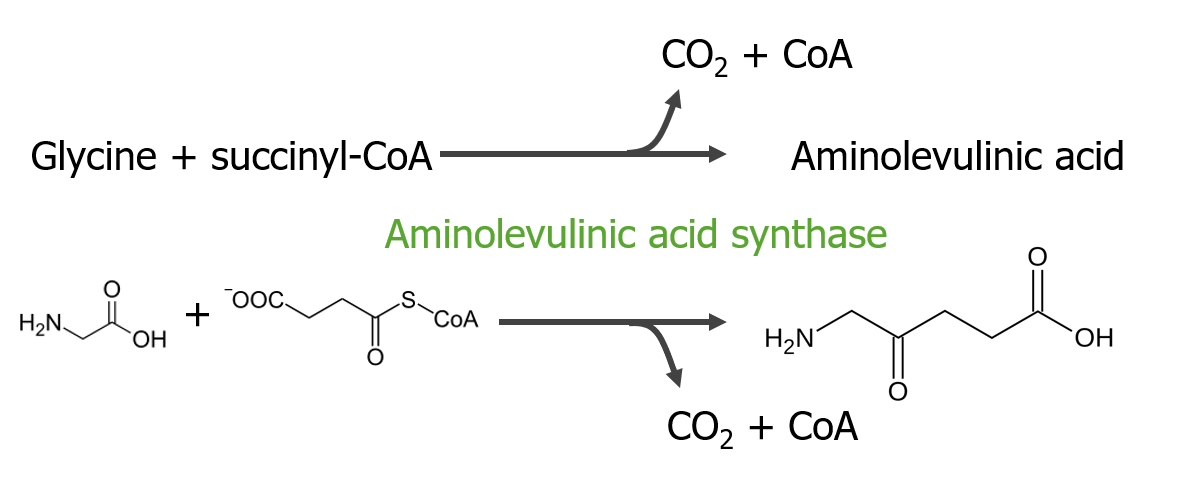
Step 1 of heme metabolism
Image by Lecturio.Step 2 is the formation of porphobilinogen (PBG).
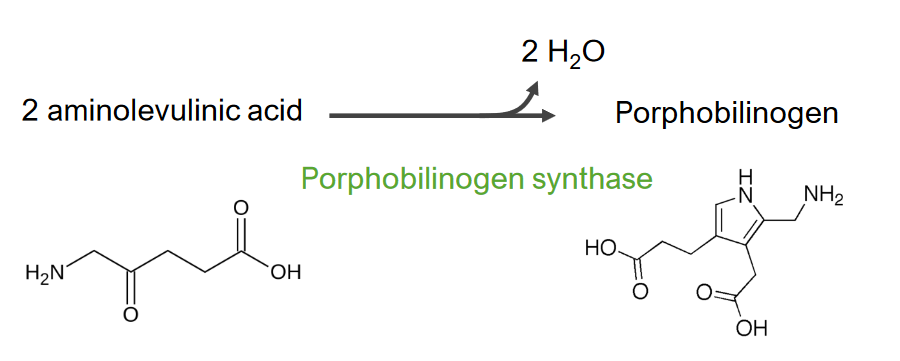
Step 2 of heme metabolism
Formation of porphobilinogen
Step 3 is the formation of hydroxymethylbilane ( HMB HMB Excessive menstrual blood loss (objectively defined as > 80 mL blood loss/cycle). Can be based on heavy flow, as determined by the patient Abnormal Uterine Bleeding).
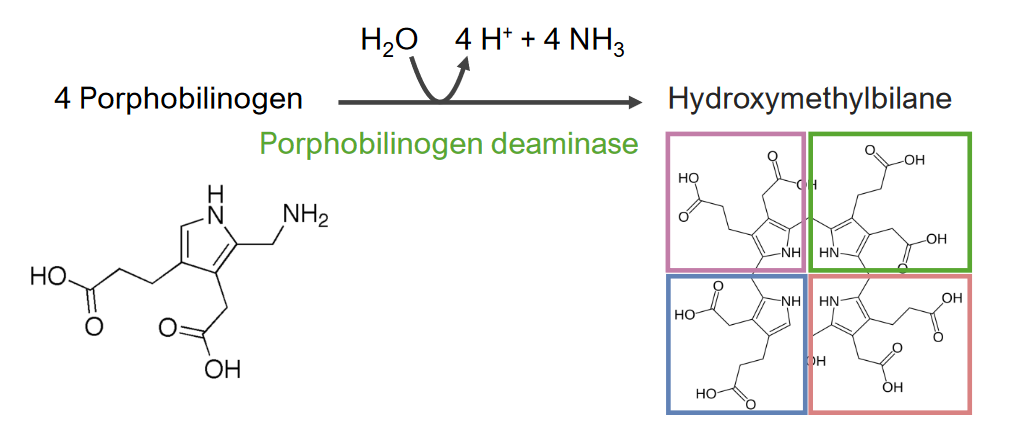
Step 3 of heme metabolism:
Formation of hydroxymethylbilane
Step 4 is the formation of uroporphyrinogen (UPG).
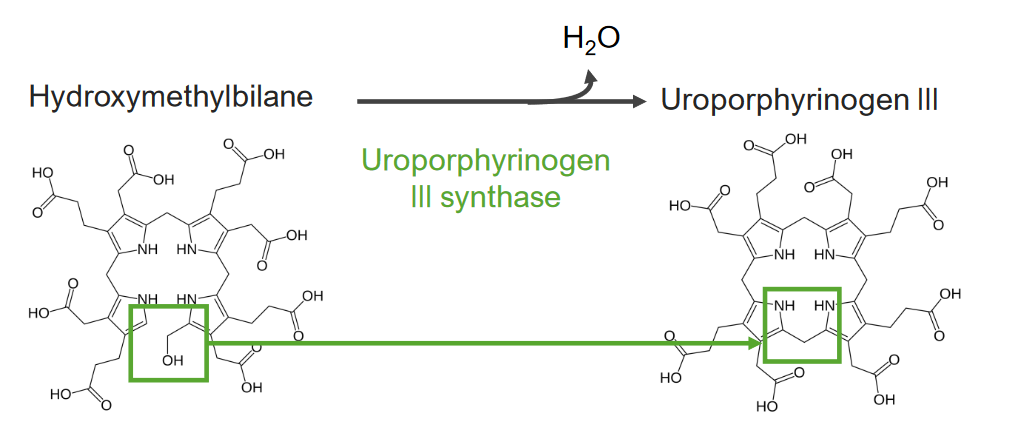
Step 4 of heme metabolism:
Formation of uroporphyrinogen
Step 5 is the synthesis Synthesis Polymerase Chain Reaction (PCR) of coproporphyrinogen (CPG) III.
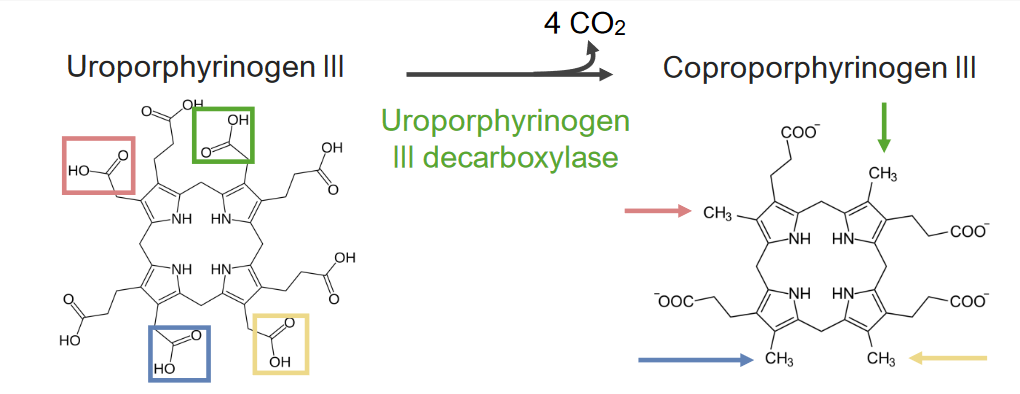
Step 5 of heme metabolism:
Formation of coproporphyrinogen III
Step 6 is the synthesis Synthesis Polymerase Chain Reaction (PCR) of protoporphyrinogen (PPG).
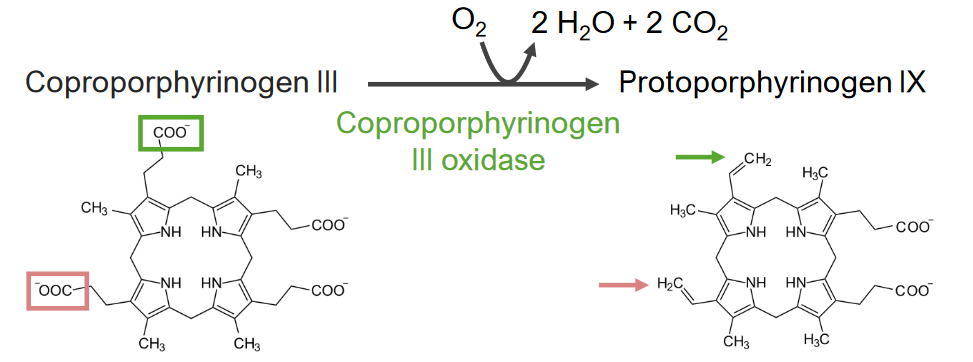
Step 6 of heme metabolism:
Synthesis of protoporphyrinogen
Step 7 is the generation of protoporphyrin (PP).
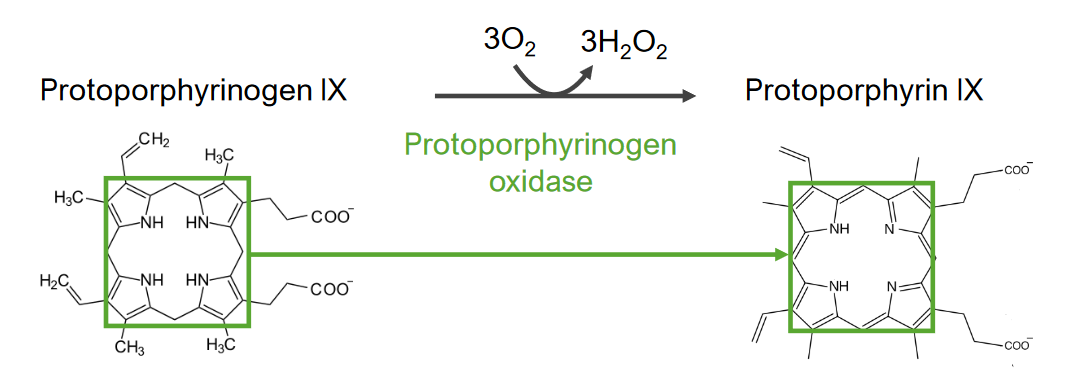
Step 7 of heme metabolism:
Generation of protoporphyrin from protoporphyrinogen IX
Step 8 is the generation of heme.
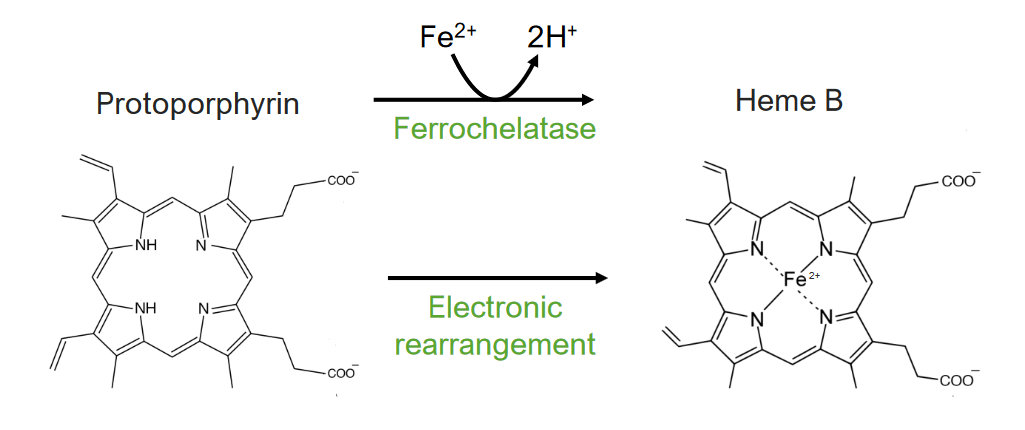
The 8th and final step of heme metabolism:
Formation of heme
| Step | Site of process | Enzyme | Disease associated with enzyme gene Gene A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. Basic Terms of Genetics mutations |
|---|---|---|---|
| 1. Synthesis Synthesis Polymerase Chain Reaction (PCR) of aminolevulinic acid | Mitochondria Mitochondria Semiautonomous, self-reproducing organelles that occur in the cytoplasm of all cells of most, but not all, eukaryotes. Each mitochondrion is surrounded by a double limiting membrane. The inner membrane is highly invaginated, and its projections are called cristae. Mitochondria are the sites of the reactions of oxidative phosphorylation, which result in the formation of ATP. They contain distinctive ribosomes, transfer RNAs; amino Acyl tRNA synthetases; and elongation and termination factors. Mitochondria depend upon genes within the nucleus of the cells in which they reside for many essential messenger RNAs. Mitochondria are believed to have arisen from aerobic bacteria that established a symbiotic relationship with primitive protoeukaryotes. The Cell: Organelles | Aminolevulinic acid synthase |
|
| 2. Formation of porphobilinogen (PBG) | Cytosol Cytosol A cell’s cytoskeleton is a network of intracellular protein fibers that provides structural support, anchors organelles, and aids intra- and extracellular movement. The Cell: Cytosol and Cytoskeleton | Aminolevulinic acid dehydratase or PBG synthase | Aminolevulinic acid dehydratase porphyria |
| 3. Formation of hydroxymethylbilane ( HMB HMB Excessive menstrual blood loss (objectively defined as > 80 mL blood loss/cycle). Can be based on heavy flow, as determined by the patient Abnormal Uterine Bleeding) | PBG deaminase/ HMB HMB Excessive menstrual blood loss (objectively defined as > 80 mL blood loss/cycle). Can be based on heavy flow, as determined by the patient Abnormal Uterine Bleeding synthase | Acute intermittent porphyria Acute intermittent porphyria An autosomal dominant porphyria that is due to a deficiency of hydroxymethylbilane synthase in the liver, the third enzyme in the 8-enzyme biosynthetic pathway of heme. Clinical features are recurrent and life-threatening neurologic disturbances, abdominal pain, and elevated level of aminolevulinic acid and porphobilinogen in the urine. Porphyrias | |
| 4. Formation of uroporphyrinogen (UPG) | UPG III synthase | Congenital erythropoietic porphyria Congenital erythropoietic porphyria An autosomal recessive porphyria that is due to a deficiency of uroporphyrinogen III synthase in the bone marrow; also known as congenital erythropoietic porphyria. This disease is characterized by splenomegaly; anemia; photosensitivity; cutaneous lesions; accumulation of hydroxymethylbilane; and increased excretion of uroporphyrins and coproporphyrins. Porphyrias | |
| 5. Synthesis Synthesis Polymerase Chain Reaction (PCR) of coproporphyrinogen (CPG) III | UPG decarboxylase | Porphyria cutanea tarda Porphyria cutanea tarda An autosomal dominant or acquired porphyria due to a deficiency of uroporphyrinogen decarboxylase in the liver. It is characterized by photosensitivity and cutaneous lesions with little or no neurologic symptoms. Type I is the acquired form and is strongly associated with liver diseases and hepatic toxicities caused by alcohol or estrogenic steroids. Type II is the familial form. Porphyrias and hepatoerythropoietic porphyria | |
| 6. Synthesis Synthesis Polymerase Chain Reaction (PCR) of protoporphyrinogen (PPG) | Mitochondria Mitochondria Semiautonomous, self-reproducing organelles that occur in the cytoplasm of all cells of most, but not all, eukaryotes. Each mitochondrion is surrounded by a double limiting membrane. The inner membrane is highly invaginated, and its projections are called cristae. Mitochondria are the sites of the reactions of oxidative phosphorylation, which result in the formation of ATP. They contain distinctive ribosomes, transfer RNAs; amino Acyl tRNA synthetases; and elongation and termination factors. Mitochondria depend upon genes within the nucleus of the cells in which they reside for many essential messenger RNAs. Mitochondria are believed to have arisen from aerobic bacteria that established a symbiotic relationship with primitive protoeukaryotes. The Cell: Organelles | CPG oxidase Oxidase Neisseria | Hereditary coproporphyria Hereditary coproporphyria An autosomal dominant porphyria that is due to a deficiency of coproporphyrinogen oxidase in the liver, the sixth enzyme in the 8-enzyme biosynthetic pathway of heme. Clinical features include both neurological symptoms and cutaneous lesions. Patients excrete increased levels of porphyrin precursors, 5-aminolevulinate and coproporphyrins. Porphyrias |
| 7. Generation of protoporphyrin (PP) | Protoporphyrinogen oxidase Oxidase Neisseria | Variegate porphyria Variegate porphyria An autosomal dominant porphyria that is due to a deficiency of protoporphyrinogen oxidase in the liver, the seventh enzyme in the 8-enzyme biosynthetic pathway of heme. Clinical features include both neurological symptoms and cutaneous lesions. Patients excrete increased levels of porphyrin precursors, coproporphyrins and protoporphyrinogen. Porphyrias | |
| 8. Generation of heme | Ferrochelatase/heme synthase | Erythropoietic protoporphyria Erythropoietic protoporphyria An autosomal dominant porphyria that is due to a deficiency of ferrochelatase (heme synthetase) in both the liver and the bone marrow, the last enzyme in the 8-enzyme biosynthetic pathway of heme. Clinical features include mainly neurological symptoms, rarely cutaneous lesions, and elevated levels of protoporphyrin and coproporphyrins in the feces. Porphyrias |
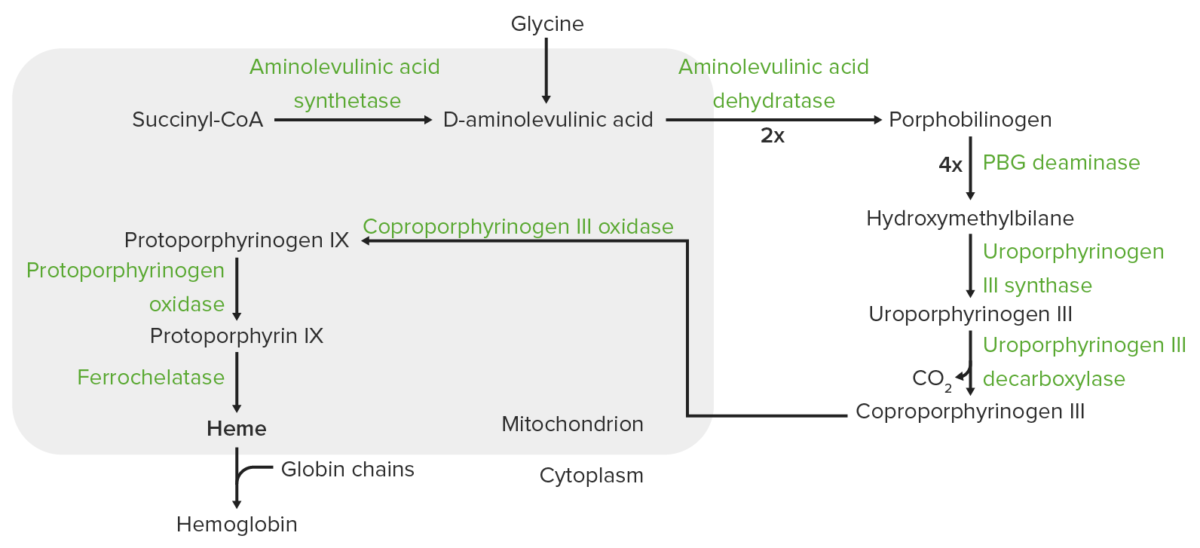
Heme synthesis:
The process of heme synthesis takes place in the mitochondria and cytoplasm.
In the mitochondria, succinyl coenzyme A (CoA) combines with glycine to form aminolevulinic acid.
This reaction is catalyzed by aminolevulinic acid synthase. The aminolevulinic acid exits to the cytoplasm, where 2 aminolevulinic acid molecules condense to produce porphobilinogen (PBG). The subsequent steps lead to the formation of coproporphyrinogen III, which is transported back to the mitochondria. Oxidase facilitates conversion of coproporphyrinogen III to protoporphyrinogen IX, which then is converted to protoporphyrin IX. Ferrous iron is inserted into protoporphyrin IX, forming heme (catalyzed by ferrochelatase).
Heme breaks down, resulting in bile Bile An emulsifying agent produced in the liver and secreted into the duodenum. Its composition includes bile acids and salts; cholesterol; and electrolytes. It aids digestion of fats in the duodenum. Gallbladder and Biliary Tract: Anatomy pigments as the end products, with bilirubin excreted through the bile Bile An emulsifying agent produced in the liver and secreted into the duodenum. Its composition includes bile acids and salts; cholesterol; and electrolytes. It aids digestion of fats in the duodenum. Gallbladder and Biliary Tract: Anatomy. The steps of heme catabolism are:
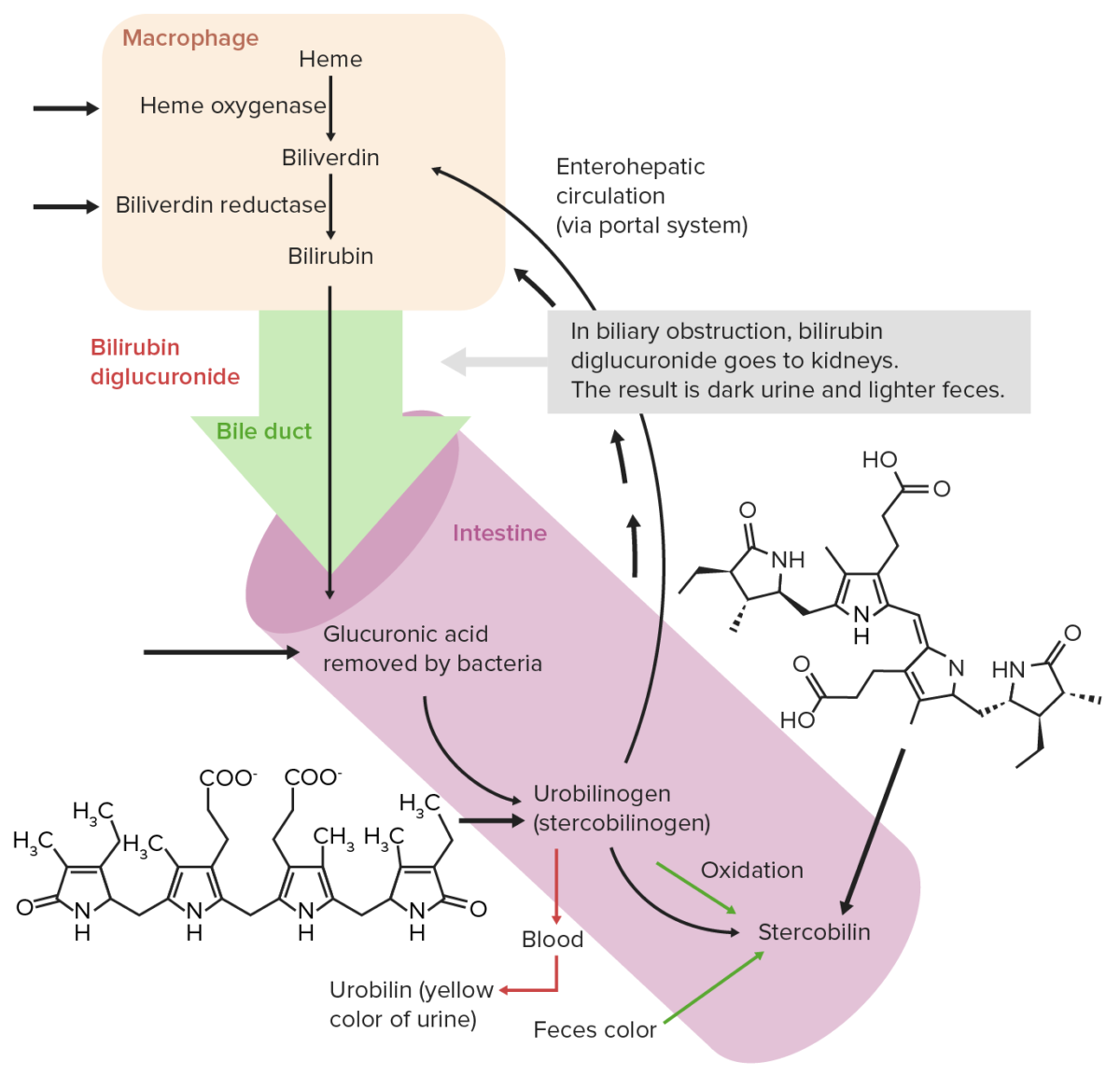
Normal extrahepatic circulation of bilirubin
Image by Lecturio. License: CC BY-NC-SA 4.0Iron absorption Iron absorption Digestion and Absorption:
Iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements transport:
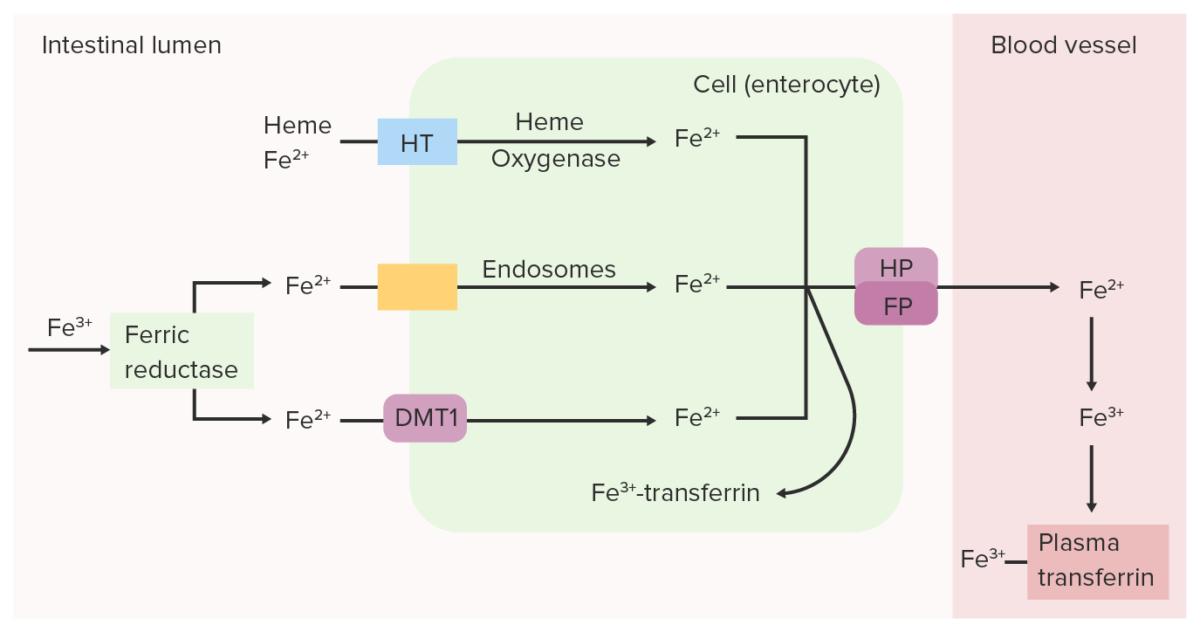
Intestinal ferric (Fe3+) reductase reduces Fe3+ (ferric) to Fe2+ (ferrous). Fe2+ is transported from the lumen into the intestinal epithelial cell through divalent metal transporter 1 (DMT1), heme transporter (HT) and/or endosomes. Fe2+ can be converted back to Fe3+ and bound to transferrin within the intestinal cell or can be transported into the blood by ferroportin (FP) and hephaestin (HP). Oxidized iron (Fe3+), which binds to plasma transferrin, is carried through the circulation to the tissues.
Image by Lecturio.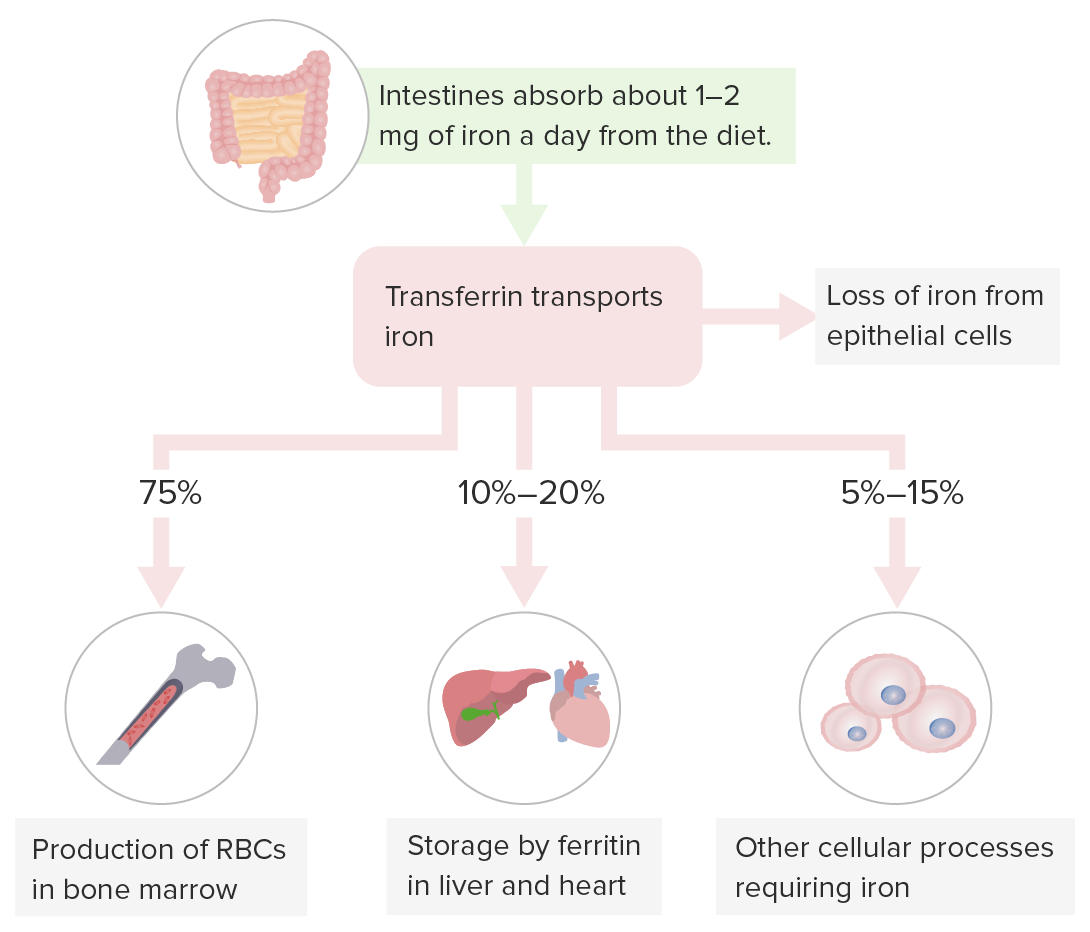
Storage of iron:
Transferrin carries iron for hematopoiesis in the bone marrow, iron storage in the liver (primary storage site) and other organs, and cellular processes requiring iron.
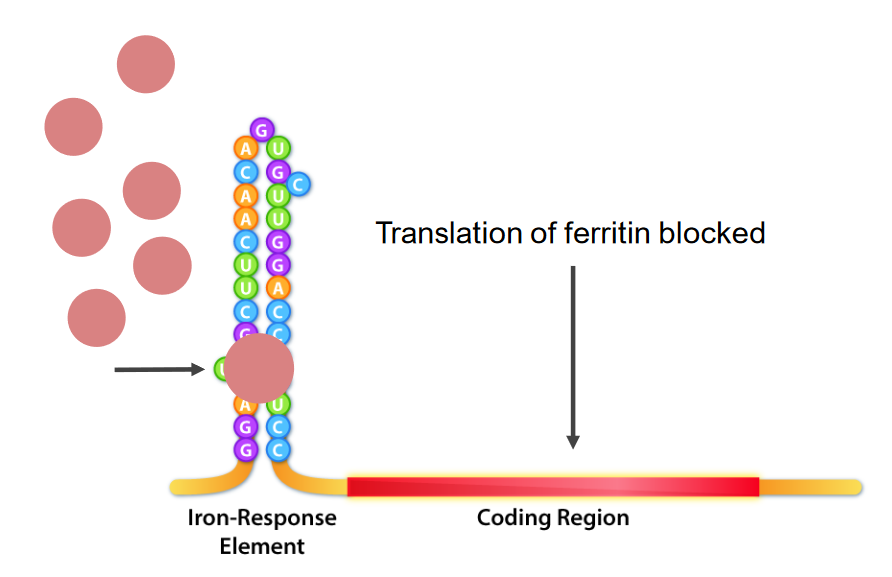
Interaction of iron-response element (IRE) binding proteins (IRBPs; pink round structures) and the IRE, which is the stem loop structure at the 5’ of the mRNA.
In iron deficiency, IRBPs bind the IRE, thus causing inhibition of ferritin mRNA translation.
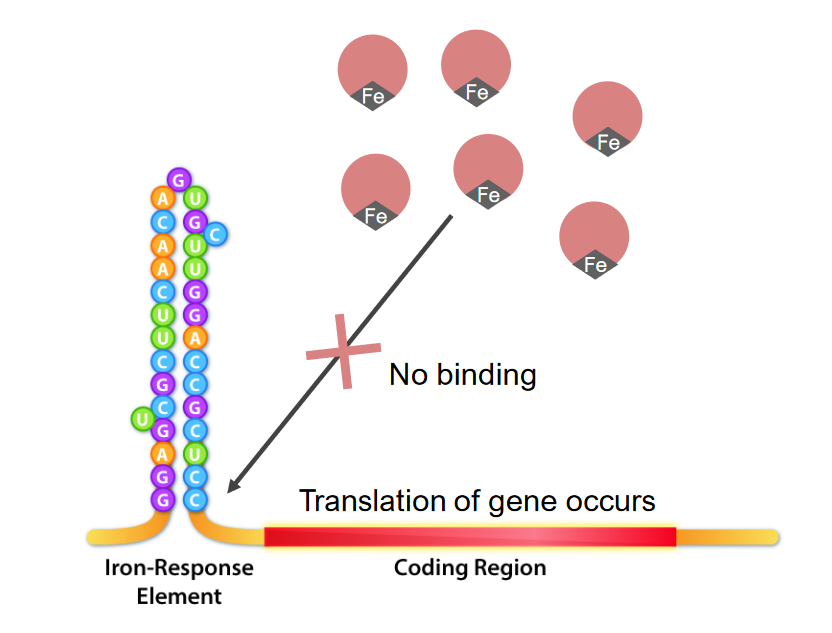
The interaction of iron response elements (IRE) and IRE binding proteins (IRBPs) in iron excess:
Iron binds the IRBPs, thus making them unavailable to bind the ferritin IRE. This allows translation to proceed.
Certain conditions require a decrease or increase in iron absorption Iron absorption Digestion and Absorption and circulating iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements, a pathway regulated by hepcidin Hepcidin Forms of hepcidin, a cationic amphipathic peptide synthesized in the liver as a prepropeptide which is first processed into prohepcidin and then into the biologically active hepcidin forms, including in human the 20-, 22-, and 25-amino acid residue peptide forms. Hepcidin acts as a homeostatic regulators of iron metabolism and also possesses antimicrobial activity. Hereditary Hemochromatosis: