Electronegativity is a measure of the ability of an atom to attract the shared electrons in a covalent bond toward itself. The electronegativity of an atom is influenced by two factors: its electron affinity and ionization energy. Bond polarity refers to the distribution of electrons between two atoms in a covalent bond. Lewis structures are diagrams that show the arrangement of atoms and electrons in a molecule. Resonance occurs when a molecule can be represented by two or more Lewis structures that differ only in the placement of electrons, and it can increase the stability of a molecule. The mesomeric effect, also known as the resonance effect, is the electronic effect that occurs when a substituent in a molecule donates or withdraws electrons through the pi-bond system. The mesomeric effect can be an important factor in determining the reactivity and properties of a molecule.
Last updated: Dec 15, 2025
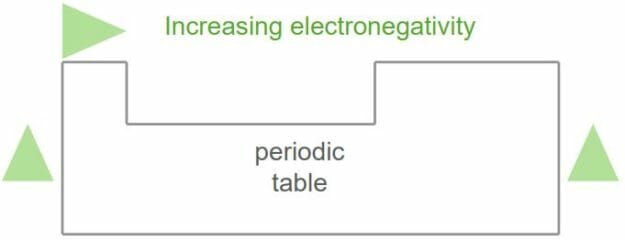
Electronegativity is defined as the tendency of an element or atom to attract bonding pairs of electrons towards itself. It also tells one the property of an atom to withdraw electron density when a covalent bond is formed. It is one of the periodic properties of the elements. This means that the elements are arranged in the periodic table so that they show a certain trend in their chemical and physical properties.
Electronegativity is a function of an atom’s electron affinity and ionization energy. These two properties indicate how strongly an atom holds its own electrons and how they attract other electrons, respectively. Electronegativity increases from left to right (metals to non-metals) across the periodic table. For the s and p block elements, the electronegativity increases as you go up a group. The most electronegative element in the periodic table is Fluorine.
Large atoms have a low affinity because:
Going across the periodic table, the affinity for bonding electrons increases because the nuclear charge increases without a significant change in the distance of the valence electrons from the nucleus Nucleus Within a eukaryotic cell, a membrane-limited body which contains chromosomes and one or more nucleoli (cell nucleolus). The nuclear membrane consists of a double unit-type membrane which is perforated by a number of pores; the outermost membrane is continuous with the endoplasmic reticulum. A cell may contain more than one nucleus. The Cell: Organelles.
Affinity of negative charge:
This affinity is described as electronegativity, and it is measured on the Pauling scale.
A critical property of molecules that depends on electronegativity is bond polarity. When two atoms form a covalent bond, the electronegativity values of the atoms involved in forming the bond dictate the polarity of the bond formed. Bonds formed can be polar or non-polar.
Polar bonds form when two atoms of different electronegativity values combine. For example, the compound hydrogen fluoride Fluoride Inorganic salts of hydrofluoric acid, hf, in which the fluorine atom is in the -1 oxidation state. Sodium and stannous salts are commonly used in dentifrices. Trace Elements, HF, is a polar compound because the bond between the H and F atoms is a polar bond. The H atom has an electronegativity value of 2.2, while an F atom has an electronegativity of 4.0. In the HF molecules, since the F atom has a higher electronegativity value than H, it has a higher tendency to attract the bond’s electron density towards itself, causing it to be slightly negative. H, on the other hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy, because of the pull of F electrons, becomes less negative or somewhat positive.
The same goes for water molecules. Water contains two OH bonds. Oxygen is a more electronegative element than hydrogen. Therefore, the covalent bond between these two atoms is polar, with the O atom being the negative end and the H atom as the positive end.
A different scenario occurs in the compound O2. The molecule only contains two oxygen atoms connected by a covalent bond. Since the two O atoms have the same electronegativity value, the two atoms will have equal strength in pulling electron densities towards them. Because of this, there will be no apparent poles in the compound, and the compound is considered non-polar.
Bond polarity affects how a compound will interact with other compounds. In terms of solubility, polar compounds can dissolve in other polar compounds. On the other hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy, non-polar substances can dissolve in other non-polar substances.
For polar compounds, dissolution occurs because of the electrostatic interaction between the positive and negative ends of molecules. The electropositive end of one molecule readily attracts the electronegative end of another molecule.
For example, considering the water molecule, the polarity arises with the O atoms at the negative end while the H atoms are at the positive ends. When two water molecules interact, the H atoms of one molecule are attracted to the O atom of the other molecule. The special type of bond that is formed is known as a Hydrogen bond.
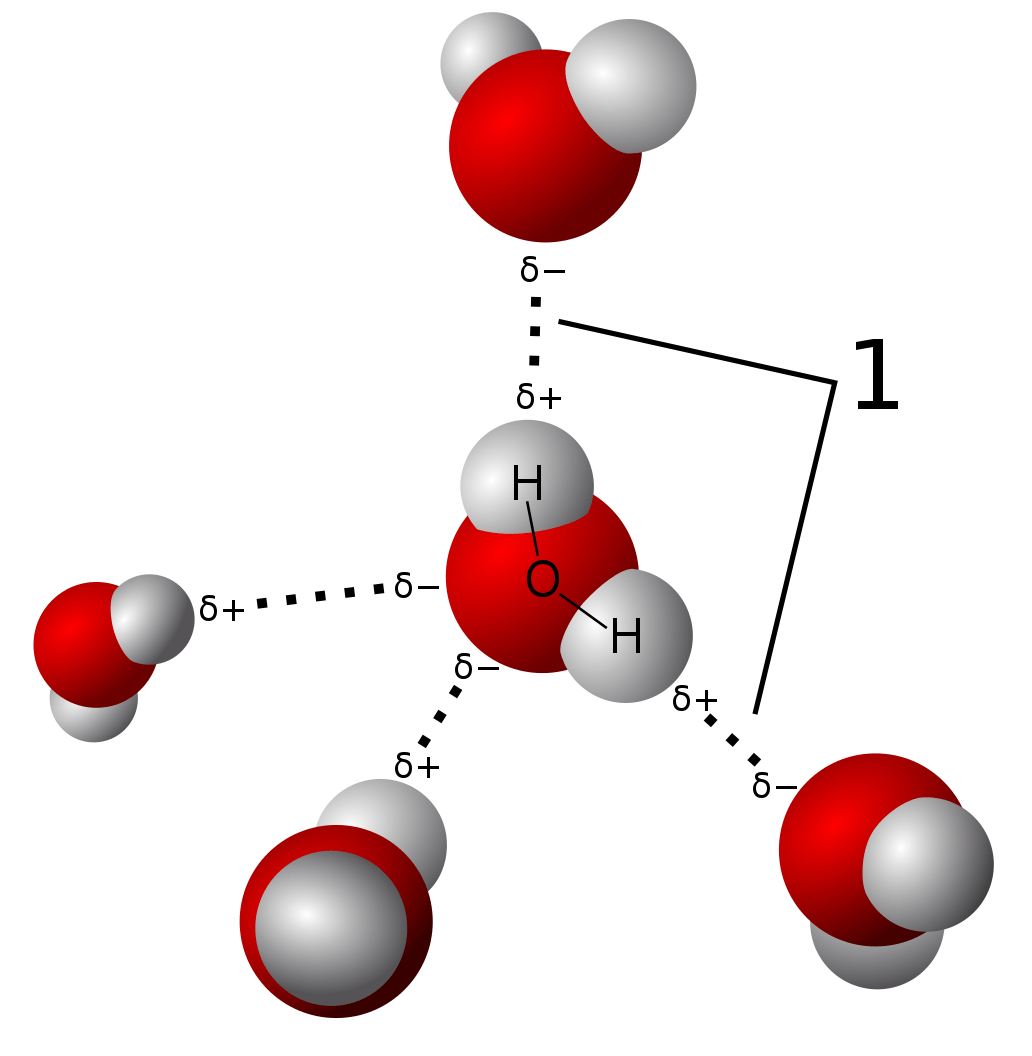
3D model of hydrogen bonds in water
Image: “Model of hydrogen bonds in water” by Qwerter. License: Public DomainResonance is the occurrence of multiple Lewis structures for a given molecule brought about by the movement of electrons among atoms in a compound. The movement of electrons is dictated by the electronegativity value of the atoms involved in the Lewis structure.
Resonance involves the rearrangement of pi and sigma bonds within a molecule. Technically, the molecule is unchanged; the same atoms are connected together. The main difference is the arrangement of single, double, and triple bonds. The molecular formulas, as well as the total number of electrons and over-all charge, will still be the same.
For example, the deprotonated form of acetic acid will exhibit resonance. Initially, the negative charge is localized in one of the oxygen atoms. However, because of the other oxygen atoms’ innate electronegative properties, the negative charge tends to attract the localized charge towards itself. This is made possible by the rearrangement of the pi bonds.
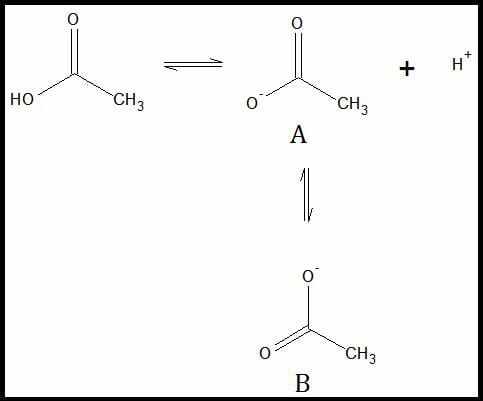
Resonance Structure of acetate ion
Image by Mark Xavier Bailon.Structures A and B are resonance structures of each other. They have the same molecular formulae with different arrangements of pi and sigma bonds. The figure below illustrates the rearrangement.
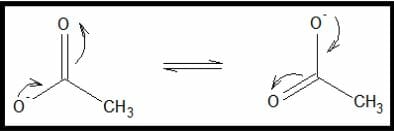
Electron rearrangement in acetate
Image by Mark Xavier Bailon.The effect of resonance is important in different compounds. It increases the compounds’ stability towards electrophilic or nucleophilic interaction. For example, since the negative charge in the acetate anion is “transferred” from one oxygen atom to another, the ion loses its ability to donate its extra electrons or the negative charge in a reaction. The negative charge’s delocalization reduces the molecule’s reactivity.
Another form of delocalization of charges is through induction, also called the inductive effect. The inductive effect is the electronic effect of an atom or a group of atoms to adjacent bonds in a molecule. Groups that cause inductive effects are usually remote (not adjacent) to the reactive side. Unlike in resonance, where actual delocalization of charges occurs, there is only an apparent delocalization in an inductive effect because of the pull of electron density by the electron-withdrawing group.
For example, consider chloroacetic acid. The only difference between chloroacetic acid and acetic acid is that a chlorine group replaces one alkyl hydrogen atom. Since a chlorine atom is more electronegative than a hydrogen atom, chlorine has a higher tendency to attract electrons towards itself.
The chlorine atom tends to pull the electron density of the C–Cl bond, leaving the C1 atom as slightly “positive.” The C1 atom then has a tendency to pull the electron density from C2, then C2. The more the electron-withdrawing group (electronegative groups) causes induction, the more stable the acetate molecule becomes. For example, replacing another H atom with a Cl atom in the chloroacetate ion increases the compound’s stability due to stronger delocalization.
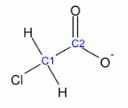
Chloroacetate ion
Image by Mark Xavier Bailon.Sometimes, both resonance and inductive effects are present in the same molecule. Some functional groups can have one type of inductive effect and the opposite resonance effect.
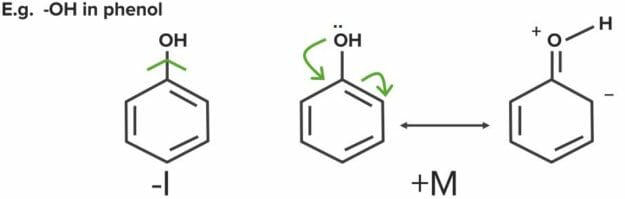
Combinations of mesomeric and inductive effects
Image by Lecturio.