The citric acid cycle, also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle, is a cyclic set of reactions that occurs in the mitochondrial matrix. The TCA cycle is the continuation of any metabolic pathway that produces pyruvate Pyruvate Derivatives of pyruvic acid, including its salts and esters. Glycolysis, which is converted into its main substrate Substrate A substance upon which the enzyme acts. Basics of Enzymes, acetyl-CoA. The TCA cycle oxidizes acetyl-CoA and produces 2 CO2, GTP, 3 NADH + H+, and FADH2. Its end products (NADH + H+ and FADH2) are passed into the electron transport chain Electron transport chain The electron transport chain (ETC) sends electrons through a series of proteins, which generate an electrochemical proton gradient that produces energy in the form of adenosine triphosphate (ATP). Electron Transport Chain (ETC) to yield a total of 10 ATP per cycle.
Last updated: Apr 25, 2025
Production of energy
Amphibolic center
The citric acid cycle provides precursors for many catabolic and anabolic processes.
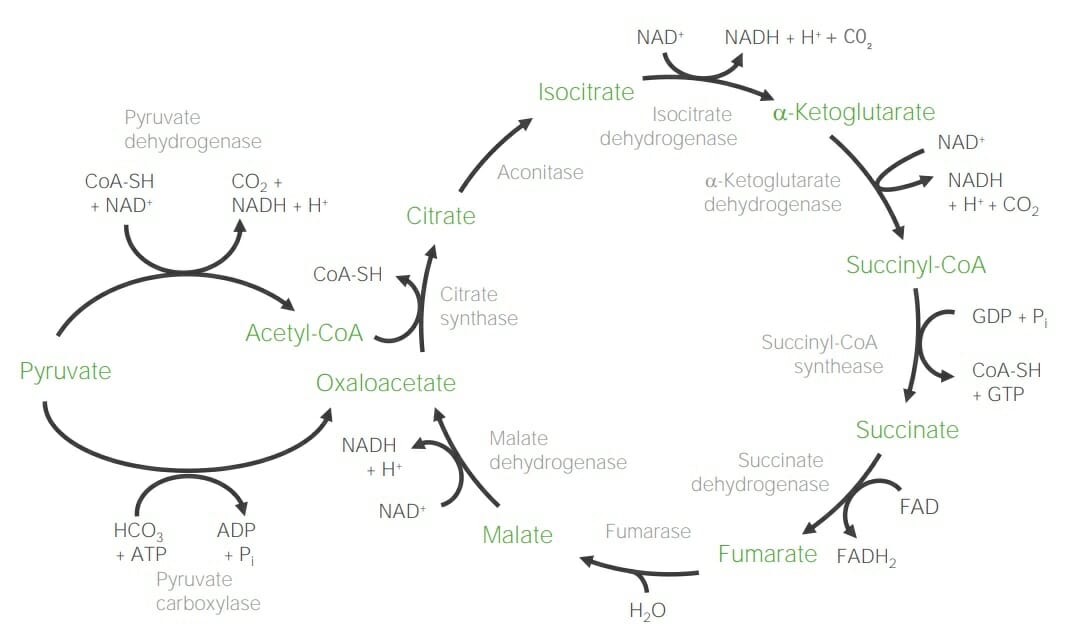
Schematic diagram of the citric acid cycle
Two main substrates: acetyl-CoA and oxaloacetate
Steps:
Acetyl-CoA (C2) + oxaloacetate (C4) → Citrate (C6) → Isocitrate (C6) → α-Ketoglutarate (C5) → Succinyl-CoA (C4) → Succinate (C4) → Fumarate (C4) → Malate (C4) → Oxaloacetate (C4)
Mnemonic device: Citrate Is Krebs’ Starting Substrate For Making Oxaloacetate
Notable reactions:
Yield:
3 NADH + H+ + 1 FADH2 + 1 GTP + 2 CO2 per acetyl-CoA (x 2 per
glucose
Glucose
A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement.
Lactose Intolerance)
Energy balance:
7.5 ATP (3 NADH + H+) + 1.5 ATP (1 FADH2) + 1 ATP (1 GTP) = 10 ATP per acetyl-CoA (x 2 per
glucose
Glucose
A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement.
Lactose Intolerance)
| Enzyme | Activation by | Inhibition by |
|---|---|---|
| Pyruvate Pyruvate Derivatives of pyruvic acid, including its salts and esters. Glycolysis dehydrogenase |
|
|
| Citrate synthase |
|
|
| Isocitrate dehydrogenase (biggest impact on the citric acid cycle) |
|
|
| α-ketoglutarate dehydrogenase | Ca CA Condylomata acuminata are a clinical manifestation of genital HPV infection. Condylomata acuminata are described as raised, pearly, flesh-colored, papular, cauliflower-like lesions seen in the anogenital region that may cause itching, pain, or bleeding. Condylomata Acuminata (Genital Warts)2+ |
|
| Succinate dehydrogenase | Succinate | Oxaloacetate |
The following process is stimulated by the tricarboxylic acid (TCA) cycle:
The following conditions inhibit the TCA cycle: