Alcohols are organic compounds that contain a hydroxyl (-OH) group attached to a carbon atom. They are named using the -ol suffix and are classified based on the number of carbon atoms attached to the carbon atom that bears the -OH group. Alcohols have higher boiling points than alkanes due to the presence of intermolecular hydrogen bonding Hydrogen bonding A low-energy attractive force between hydrogen and another element. It plays a major role in determining the properties of water, proteins, and other compounds. DNA Types and Structure between the hydroxyl groups. Alcohols are also more polar than alkanes, which leads to higher solubility in polar solvents. The reactivity of alcohols is also different from alkanes due to the presence of the -OH group, which makes them more reactive in reactions such as oxidation, dehydration Dehydration The condition that results from excessive loss of water from a living organism. Volume Depletion and Dehydration, and esterification Esterification The process of converting an acid into an alkyl or aryl derivative. Most frequently the process consists of the reaction of an acid with an alcohol in the presence of a trace of mineral acid as catalyst or the reaction of an Acyl chloride with an alcohol. Esterification can also be accomplished by enzymatic processes. Lipid Metabolism. Alcohols can be oxidized to aldehydes, ketones Ketones Organic compounds containing a carbonyl group =C=O bonded to two hydrocarbon groups. Basics of Carbohydrates, or carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance depending on the oxidizing agent used. The biological oxidation of alcohol requires the presence of a coenzyme, such as NAD NAD+ A coenzyme composed of ribosylnicotinamide 5'-diphosphate coupled to adenosine 5'-phosphate by pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). Pentose Phosphate Pathway+ or FAD.
Last updated: Dec 15, 2025
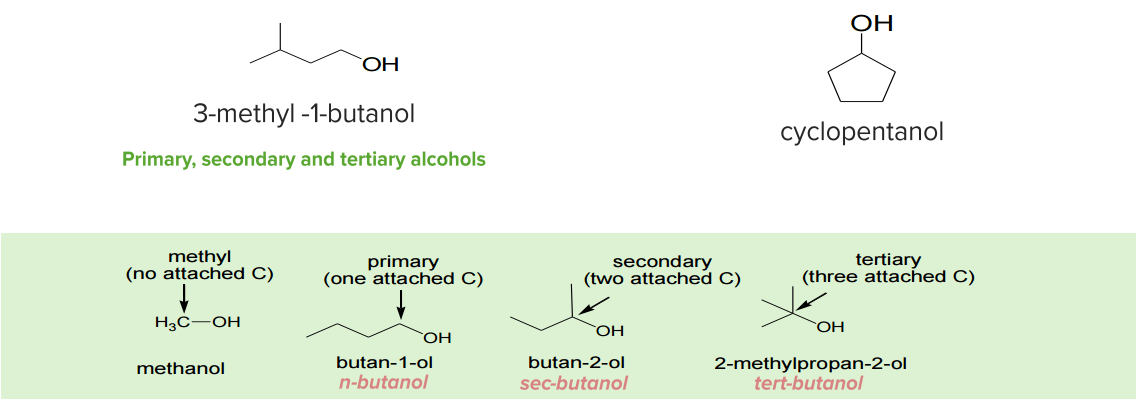
Primary, secondary, and tertiary alcohols
Image by Lecturio.Alcohol is an organic compound containing a hydroxyl (R-OH) group. It may be classified as primary (1°), secondary (2°) and tertiary (3°) based on where the hydroxyl group is attached. Primary alcohols are alcohols where the hydroxyl carbon is bound to one other carbon atom. In secondary alcohols, the hydroxyl carbon is attached to two other carbon atoms, and in tertiary alcohols, they are attached to three other carbon atoms. Below are the general structures of these three types of alcohol:
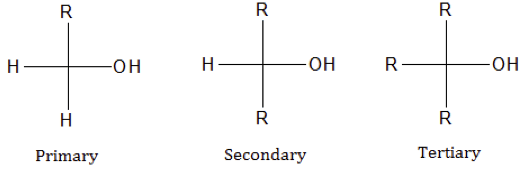
Classes of alcohols
Image by Mark Xavier Bailon.Compared to an alkane, alcohol has a much higher boiling point than the alkane of similar molecular weights. This is because of the different governing intermolecular forces of attraction (IMF) for the two compounds. For alkane, the major IMF present is only the London Dispersion Dispersion Central tendency is a measure of values in a sample that identifies the different central points in the data, often referred to colloquially as “averages.” The most common measurements of central tendency are the mean, median, and mode. Identifying the central value allows other values to be compared to it, showing the spread or cluster of the sample, which is known as the dispersion or distribution. Measures of Central Tendency and Dispersion Forces.
This is because of the compound’s non-polar nature. However, for alcohol, the -OH group helped the compound exhibit much stronger IMF in the form of hydrogen bonding Hydrogen bonding A low-energy attractive force between hydrogen and another element. It plays a major role in determining the properties of water, proteins, and other compounds. DNA Types and Structure.
Hydrogen Bonds (H-bonds) form when a Hydrogen atom binds to the small, highly electronegative atoms, such as nitrogen Nitrogen An element with the atomic symbol n, atomic number 7, and atomic weight [14. 00643; 14. 00728]. Nitrogen exists as a diatomic gas and makes up about 78% of the earth’s atmosphere by volume. It is a constituent of proteins and nucleic acids and found in all living cells. Urea Cycle, oxygen, and fluorine, and forms an electrostatic attraction to another nearby highly electronegative atom (F, O, N). Because of the strong interaction between the H atom and the electronegative atom, alcohol molecules are more attracted to each other and have a higher boiling point than alkanes.
Note: A hydrogen bond is a special type of attractive interaction that exists between an electronegative atom and a hydrogen atom bonded to another electronegative atom.
It has only 5%–10% of a covalent bond’s strength, but it has a profound effect on the boiling point and solubility of alcohols.
The cells of living things are made up of many different sorts of molecules. Two important classes of molecules are nucleic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance and proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis. Parts of these very large molecules are involved in hydrogen bonds with other parts of the same molecules. This is very important in establishing the molecules’ structures and properties. The double heliac structure of DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure, for example, is mainly due to hydrogen bonding Hydrogen bonding A low-energy attractive force between hydrogen and another element. It plays a major role in determining the properties of water, proteins, and other compounds. DNA Types and Structure between the base pairs. Two strands of complementary bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance are bonded together through the hydrogen bond, which enables replication.
Note: For example, hydrogen bonding Hydrogen bonding A low-energy attractive force between hydrogen and another element. It plays a major role in determining the properties of water, proteins, and other compounds. DNA Types and Structure plays an important role in determining the three-dimensional secondary structures of proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis.
Hydrogen bonds form between backbone oxygen and amide hydrogen to form more frequently either the a-helix or the b-sheet secondary structure of proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis.
Boiling points (b.p.): more energy is needed to break the hydrogen bonds between molecules, so the b.p. is higher than in an alkane of similar M.W.
| Nr. of C | Alkane | b.p. (°C) | Alcohol | b.p. (°C) |
|---|---|---|---|---|
| 1 | CH4 | –161 | CH3OH | 64.7 |
| 2 | CH3CH3 | –88 | CH3CH2OH | 78 |
| 3 | CH3CH2CH3 | –42 | CH3(CH2)2OH | 97 |
| 4 | CH3(CH2)2CH3 | –0.5 | CH3(CH2)3OH | ≈ 116 |
| 5 | CH3(CH2)3CH3 | ≈ 35 | CH3(CH2)4OH | ≈ 136 |
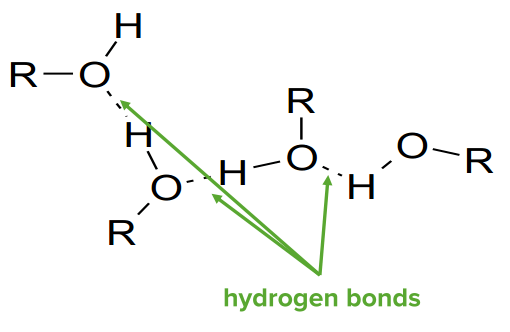
Hydrogen bonds
Image by Lecturio.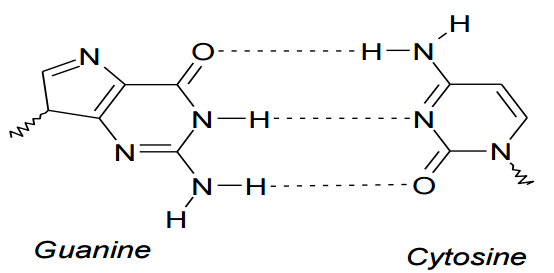
Guanine and cytosine
Image by Lecturio.Because of the inherent polarity of alcohol, this organic compound family is considered more reactive than its hydrocarbon counterpart. One of the essential reactions of alcohol is its reaction with a hydrogen halide to produce an alkyl halide and water.
R-OH + H-X → R-X + H-OH
Different types of hydrogen halides have different reactivities for this reaction. The reactivity of hydrogen halides parallels their acidity. This means HI, being the strongest acid, will be the most reactive, and HF, being the weakest acid, will be the least reactive.
Reactivity: HI > HBr > HCl HCL Hairy cell leukemia (HCL) is a rare, chronic, B-cell leukemia characterized by the accumulation of small mature B lymphocytes that have “hair-like projections” visible on microscopy. The abnormal cells accumulate in the peripheral blood, bone marrow (causing fibrosis), and red pulp of the spleen, leading to cytopenias. Hairy Cell Leukemia >> HF
The reaction kinetics also depend on the type of alcohol used in the reaction. Different alcohol types also have different reactivities. The reactivity of alcohols with hydrogen halides are shown below.
Methyl (CH3OH) < Primary (RCH3OH) < Secondary (R2CHOH) < Tertiary (R2COH)
Tertiary alcohols produce high yields in less time than other alcohol types. To increase the rates of reactions for secondary and primary alcohols, the scientist usually performs the reaction at elevated temperatures.
The reaction of alcohol and a hydrogen halide is a substitution reaction. The halogen group replaces the –OH group in the structure. The reaction proceeds through three elementary steps. The first step involves the attachment of the acidic proton to the oxygen atom of the hydroxyl group. This intermediate is highly unstable. Thus, step 2 consists of the removal of a water molecule from the alkyloxonium ion, producing a carbocation intermediate. On the last step, the chloride Chloride Inorganic compounds derived from hydrochloric acid that contain the Cl- ion. Electrolytes ion attaches itself to the carbonation, producing the final alkyl halide. As an example, consider the reaction of 2-propanol and HBr at an elevated temperature.
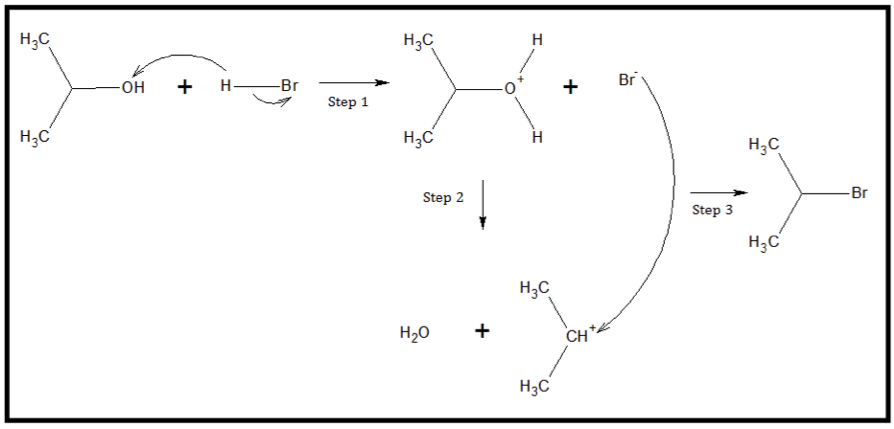
Reaction of 2-propanol and HBr to produce 2-bromopropane
Image by Mark Xavier Bailon.Another important reaction of alcohol is in the production of alkenes. The reaction proceeds once water is eliminated. Except for primary alcohols, reactions, just like in the other alkene preparation method, are regioselective; that is, a mixture of products will form, but one will form in a larger amount. Even though a mixture of products will form, alcohol’s dehydration Dehydration The condition that results from excessive loss of water from a living organism. Volume Depletion and Dehydration reaction is stereoselective; that is, it favors the production of the most stable stereoisomer.
Alcohol dehydration Dehydration The condition that results from excessive loss of water from a living organism. Volume Depletion and Dehydration is catalyzed by strong acids Strong acids Acid-Base Balance, like H2SO4, and heating. The reaction also requires three steps. Step 1 involves a proton from the acid attaching to the O atom of the –OH group, forming the alkoxonium ion. Step 2 involves the dehydration Dehydration The condition that results from excessive loss of water from a living organism. Volume Depletion and Dehydration, or removal of a water molecule from the alkyloxonium ion, leaving a carbocation intermediate. The carbocation intermediate then participates as a strong Bronsted acid, donating one H+ to a water molecule and regenerating the original proton used up in step 1. Below is the mechanism of an alkene formation through an acid-catalyzed dehydration Dehydration The condition that results from excessive loss of water from a living organism. Volume Depletion and Dehydration reaction of 3-propanol.
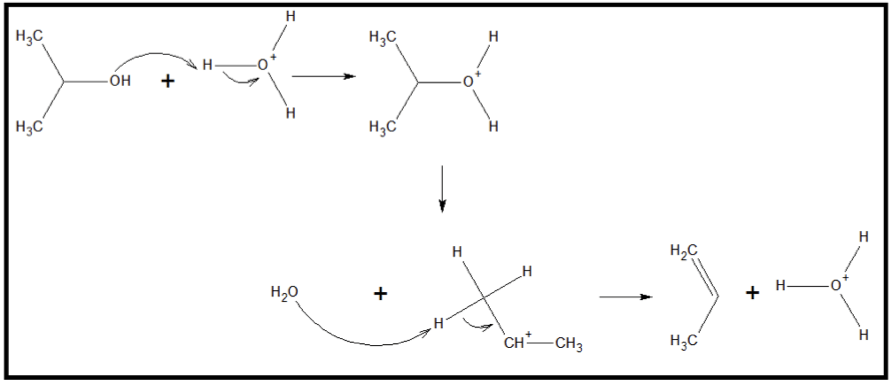
Acid-catalyzed dehydration reaction of 2-propanol
Image by Mark Xavier Bailon.Alcohol is also important in the preparation of esters. Esters are compounds produced by the acid-catalyzed condensation of the alcohol and a carboxylic acid. The general reaction is reversible but favors the products when simple alcohols and carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance are used. The number of alkyl substituents of alcohol affects its reactivity in an esterification Esterification The process of converting an acid into an alkyl or aryl derivative. Most frequently the process consists of the reaction of an acid with an alcohol in the presence of a trace of mineral acid as catalyst or the reaction of an Acyl chloride with an alcohol. Esterification can also be accomplished by enzymatic processes. Lipid Metabolism process. The more alkyl substituents, the less reactive it is to esterification Esterification The process of converting an acid into an alkyl or aryl derivative. Most frequently the process consists of the reaction of an acid with an alcohol in the presence of a trace of mineral acid as catalyst or the reaction of an Acyl chloride with an alcohol. Esterification can also be accomplished by enzymatic processes. Lipid Metabolism for steric reasons.
Reactivity to Esterification Esterification The process of converting an acid into an alkyl or aryl derivative. Most frequently the process consists of the reaction of an acid with an alcohol in the presence of a trace of mineral acid as catalyst or the reaction of an Acyl chloride with an alcohol. Esterification can also be accomplished by enzymatic processes. Lipid Metabolism:
Methyl (CH3OH) > Primary (RCH2OH) > Secondary (R2CHOH) > Tertiary (R3COH)
The first step in the esterification Esterification The process of converting an acid into an alkyl or aryl derivative. Most frequently the process consists of the reaction of an acid with an alcohol in the presence of a trace of mineral acid as catalyst or the reaction of an Acyl chloride with an alcohol. Esterification can also be accomplished by enzymatic processes. Lipid Metabolism process is the protonation of the carbonyl oxygen atom of the carboxylic acid. Because of the interaction between the proton and oxygen, the carbonyl carbon becomes very susceptible to a nucleophilic attack by the oxygen atom of the alcohol. The next step involves a proton transfer from the alcohol group to the –OH of the carboxylic acid. Because of the relative instability of the ion produced, water readily leaves the structure. The last step involves regenerating the proton used up in the first step. The figure below shows the general mechanism for the reaction.
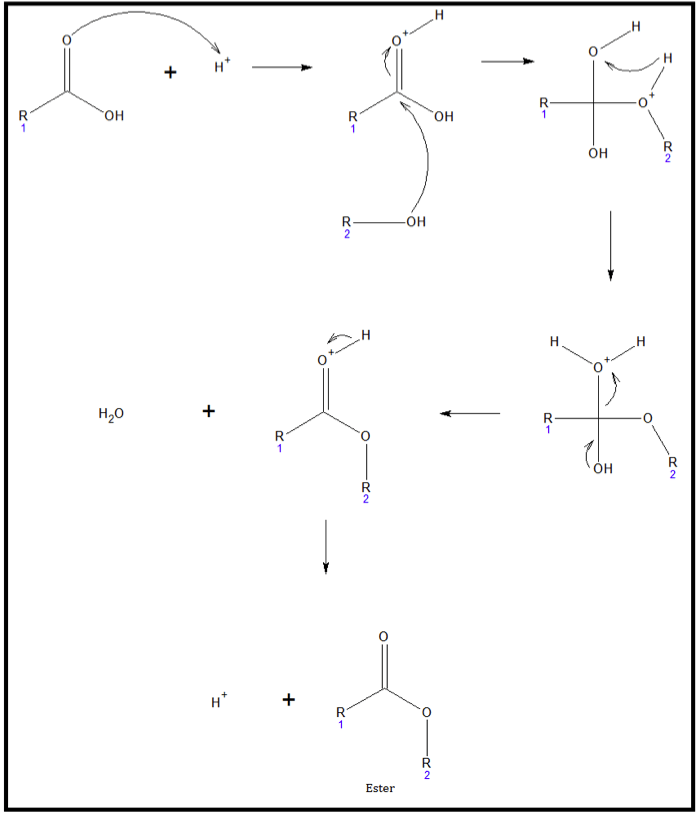
Acid-catalyzed esterification
Image by Mark Xavier Bailon.Alcohols can be converted to a carbonyl compound by reaction with an oxidizing agent. It can be converted into an aldehyde, a ketone, or a carboxylic acid. Primary alcohols may be oxidized to produce either an aldehyde or a carboxylic acid. The usual oxidizing agent for this reaction is dichromate (Cr2O72-), pyridinium chlorochromate (PCC – C5H5NH+ ClCrO3– ), C5H5NH+, and pyridinium dichromate (PDC – (C5H5NH)22+ Cr2O72-). Secondary alcohols are oxidized to ketones Ketones Organic compounds containing a carbonyl group =C=O bonded to two hydrocarbon groups. Basics of Carbohydrates using the same set of reagents. Tertiary carbons cannot undergo oxidation since they lack hydrogen on their hydroxyl-bearing carbon.
In biological systems, enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes can induce oxidation of alcohols and/or the reduction of carbonyl compounds to alcohols. For example, in the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy, ethanol Ethanol A clear, colorless liquid rapidly absorbed from the gastrointestinal tract and distributed throughout the body. It has bactericidal activity and is used often as a topical disinfectant. It is widely used as a solvent and preservative in pharmaceutical preparations as well as serving as the primary ingredient in alcoholic beverages. Ethanol Metabolism may be metabolized into acetaldehyde Acetaldehyde A colorless, flammable liquid used in the manufacture of acetic acid, perfumes, and flavors. It is also an intermediate in the metabolism of alcohol. It has a general narcotic action and also causes irritation of mucous membranes. Large doses may cause death from respiratory paralysis. Ethanol Metabolism in the presence of the enzyme alcohol dehydrogenase Alcohol dehydrogenase A zinc-containing enzyme which oxidizes primary and secondary alcohols or hemiacetals in the presence of nad. In alcoholic fermentation, it catalyzes the final step of reducing an aldehyde to an alcohol in the presence of nadh and hydrogen. Ethanol Metabolism.
Most of the time, biological oxidation of alcohol, and the reverse process, also require the presence of a coenzyme. Coenzymes Coenzymes Small molecules that are required for the catalytic function of enzymes. Many vitamins are coenzymes. Basics of Enzymes are organic compounds that work with the enzyme to bring about the chemical change in the substrate Substrate A substance upon which the enzyme acts. Basics of Enzymes. Coenzymes Coenzymes Small molecules that are required for the catalytic function of enzymes. Many vitamins are coenzymes. Basics of Enzymes have functional groups complementary to that of the substrate Substrate A substance upon which the enzyme acts. Basics of Enzymes, and the enzyme catalyzes the redox reaction in the substrate-coenzyme complex. In the process, ethanol Ethanol A clear, colorless liquid rapidly absorbed from the gastrointestinal tract and distributed throughout the body. It has bactericidal activity and is used often as a topical disinfectant. It is widely used as a solvent and preservative in pharmaceutical preparations as well as serving as the primary ingredient in alcoholic beverages. Ethanol Metabolism is oxidized, and the original coenzyme is reduced. The coenzyme used for the biological oxidation of ethanol Ethanol A clear, colorless liquid rapidly absorbed from the gastrointestinal tract and distributed throughout the body. It has bactericidal activity and is used often as a topical disinfectant. It is widely used as a solvent and preservative in pharmaceutical preparations as well as serving as the primary ingredient in alcoholic beverages. Ethanol Metabolism is an oxidized form of nicotinamide adenine dinucleotide Nicotinamide adenine dinucleotide A coenzyme composed of ribosylnicotinamide 5′-diphosphate coupled to adenosine 5′-phosphate by pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). Pentose Phosphate Pathway ( NAD NAD+ A coenzyme composed of ribosylnicotinamide 5′-diphosphate coupled to adenosine 5′-phosphate by pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). Pentose Phosphate Pathway+). When the ethanol Ethanol A clear, colorless liquid rapidly absorbed from the gastrointestinal tract and distributed throughout the body. It has bactericidal activity and is used often as a topical disinfectant. It is widely used as a solvent and preservative in pharmaceutical preparations as well as serving as the primary ingredient in alcoholic beverages. Ethanol Metabolism is converted to acetaldehyde Acetaldehyde A colorless, flammable liquid used in the manufacture of acetic acid, perfumes, and flavors. It is also an intermediate in the metabolism of alcohol. It has a general narcotic action and also causes irritation of mucous membranes. Large doses may cause death from respiratory paralysis. Ethanol Metabolism, NAD NAD+ A coenzyme composed of ribosylnicotinamide 5′-diphosphate coupled to adenosine 5′-phosphate by pyrophosphate linkage. It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). Pentose Phosphate Pathway+ is reduced to NADH.
Note: Alcohol oxidation is very important in organic synthesis Synthesis Polymerase Chain Reaction (PCR). There are different possible products, depending on whether the alcohol is 10, 20, 30.
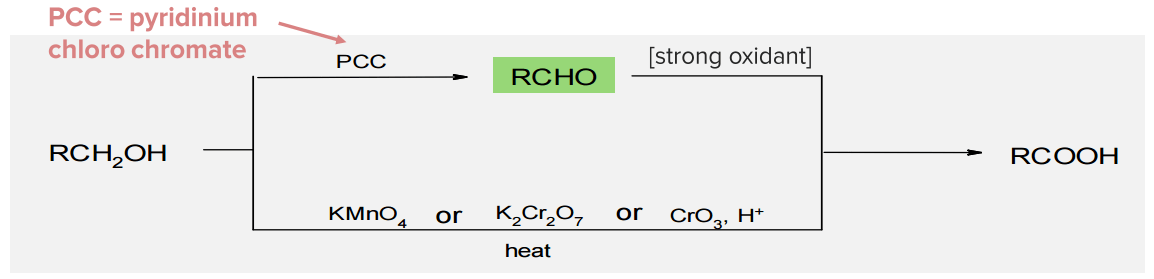
Oxidation of primary alcohols:
The product depends on reaction conditions. Note: In acidic conditions, dehydration might happen instead.
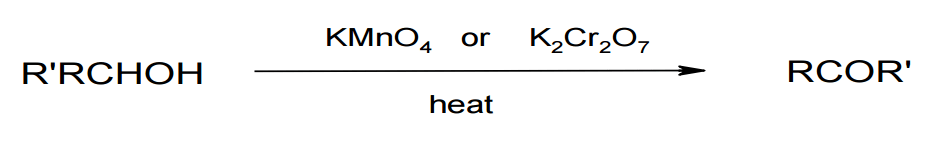
Oxidation of secondary alcohols
Image by Lecturio.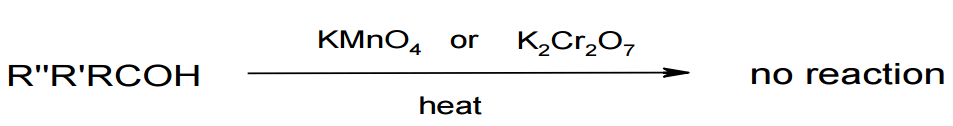
Oxidation of tertiary alcohols
Image by Lecturio.Sometimes we also have H ions along, so reduction also becomes the gain of H and oxidation the loss of H.
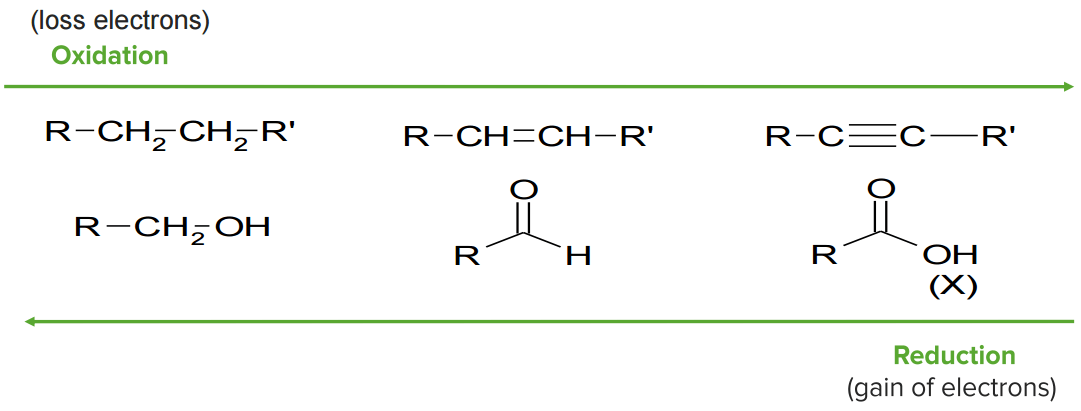
Oxidation and reduction
Image by Lecturio.