Agonism and antagonism are terms used to describe the interaction of ligands with their receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors. An agonist is a ligand that binds to a receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors and activates it. An antagonist is a ligand that binds to a receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors but does not activate it, thereby blocking the action of other ligands. Receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors are specialized proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis that are present on the surface or inside cells in the body. They bind BIND Hyperbilirubinemia of the Newborn to specific ligands, which are typically small molecules, and initiate a signaling cascade that leads to a physiological response. The binding of a ligand to a receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors is governed by several types of interactions, including covalent bonding, ionic interactions, electrostatic interactions, dipole-dipole interactions, charge-transfer complexes, hydrophobic bonding, van der Waals bonding, hydrogen bonding, and London dispersion Dispersion Central tendency is a measure of values in a sample that identifies the different central points in the data, often referred to colloquially as "averages." The most common measurements of central tendency are the mean, median, and mode. Identifying the central value allows other values to be compared to it, showing the spread or cluster of the sample, which is known as the dispersion or distribution. Measures of Central Tendency and Dispersion forces. The shape and isomerism of a ligand determine its ability to fit into the active site Active site Area of an enzyme that binds to specific substrate molecules in order to facilitate a reaction. Basics of Enzymes of a receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors and interact with it.
Last updated: Dec 15, 2025
Note: A receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors is a protein within a cell or in a cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane, which responds specifically to a particular neurotransmitter, hormone, antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination, or other substance (ligand). It usually undergoes a conformational or biochemical shift in such a way that it initiates a chain of intracellular events by which the cell reacts to the initial ligand.
Receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors are specific areas of proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis and glycoproteins Glycoproteins Conjugated protein-carbohydrate compounds including mucins, mucoid, and amyloid glycoproteins. Basics of Carbohydrates embedded in the cellular membrane or in the nuclei of living cells. Ligands may selectively bind BIND Hyperbilirubinemia of the Newborn to these receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors. Binding of ligands is significantly affected by the stereoelectronic structure of compounds which will result in specific types of interactions with the receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors.
The ligands usually selectively bind BIND Hyperbilirubinemia of the Newborn with the receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors that have complementary properties with it. When ligands attach to these receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors, a biological response – positive or negative – will occur. Positive responses involve promoting or activating physiological processes, such as the opening of ion channels Channels The Cell: Cell Membrane. Negative responses occur if the reverse happens. The binding of the ligand to the receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors causes inhibition of physiological processes.
One common ligand is pharmaceutical drugs. Each drug is a ligand for a particular receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors. And drugs, depending on their properties, can be agonistic or antagonistic of a receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors and the specific physiological processes associated with it. Drugs that result in a positive physiological response are called agonists, while drugs that bind BIND Hyperbilirubinemia of the Newborn to the receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors but do not cause a response or cause the inhibition of a response, are called antagonists. For example, muscle relaxants bind BIND Hyperbilirubinemia of the Newborn to cholinergic receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors, and a naturally occurring agonist at this class of receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors is acetylcholine Acetylcholine A neurotransmitter found at neuromuscular junctions, autonomic ganglia, parasympathetic effector junctions, a subset of sympathetic effector junctions, and at many sites in the central nervous system. Receptors and Neurotransmitters of the CNS:
Other active sites where drugs can bind BIND Hyperbilirubinemia of the Newborn include enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes. This apparent contradiction was resolved by the introduction of the idea of a receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors. A receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors is a site where a drug binds and then brings about a physical response.
Drugs are recognized by their targets via various types of interaction. Drugs binding at the same site but in a different way can give rise to different effects (e.g. agonists and antagonists). Knowledge of these interactions allows us to work out how drugs bind BIND Hyperbilirubinemia of the Newborn, design new drugs, and predict how they will bind BIND Hyperbilirubinemia of the Newborn.
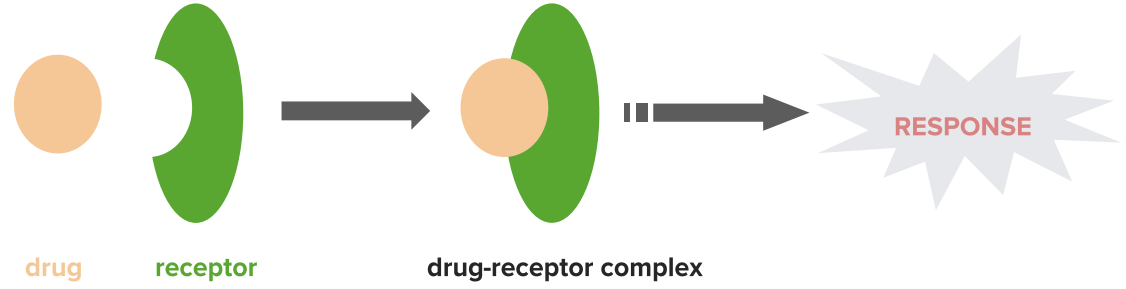
Mechanism of agonists
Image by Lecturio.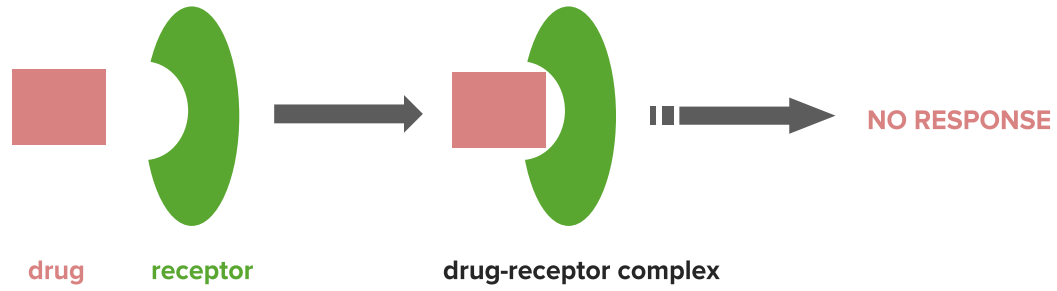
Mechanism of antagonists
Image by Lecturio.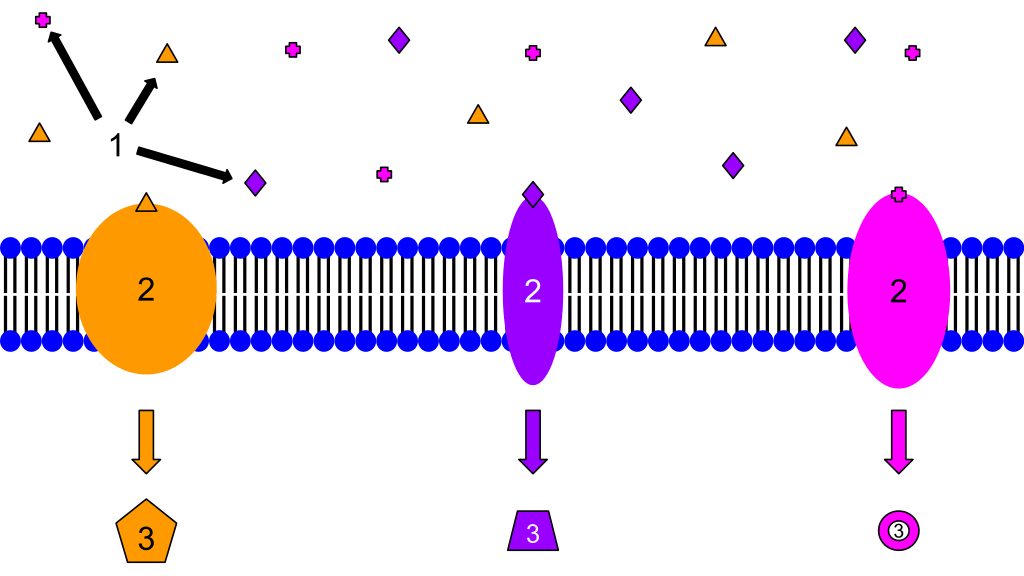
Membrane receptors:
1. Ligands
2. Receptors
3. Secondary Messengers
These are examples of membrane receptors. Typically, they are proteins that are embedded in the membrane. Although there are many different ligands located outside of the cell, membrane proteins are specific, and only certain ligands will bind to each one. That is why each protein has a different ligand and also induces a different cellular response. The response may be a transcription of a gene, cell growth, or any other cellular actions
The type of binding between ligands and receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors is governed by the concept of chemical bonding. Intra- and intermolecular forces of attraction play a big role in understanding the binding chemistry between ligands and receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors. These interactions include covalent bonding, ionic bonding, and dipole-dipole interactions. When the ligand approaches the receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors and is within an appropriate distance, a bond is formed and the drug’s mechanism of action occurs (e.g. agonism or antagonism).
A small number of drugs can also make covalent bonds with their targets. Covalent bonds are strong and hence drugs forming them will usually be permanently bound to their target. Some anti-cancer drugs alkylate the DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure within tumor Tumor Inflammation cells. The alkylated DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure cannot function and hence the cell dies (e.g. mechlorethamine).
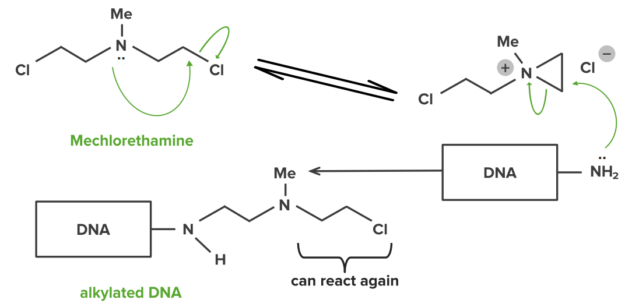
Mechlorethamine: example of DNA interstrand linking
Image by Lecturio.Ionic interactions are more reversible, compared to its covalent counterpart. This type of bond is effective at greater distances between the charges. The strength of this interaction is dependent on the distance between the charges.
| Bond type | Approx. energy (kJ/mol) |
|---|---|
| Covalent (single) | 300—450 |
| Ionic | 20—40 |
| Ion-dipole | Up to 150 |
| Hydrogen | 37 |
| Dipole-dipole | 5 |
| Hydrophobic | 4 |
| Van der Waals | 1 |
In an electronic dipole, a polarized bond is formed. In it, there is a partially positive end and a partially negative end. The interaction between the positive and negative ends of different compounds form electrostatic bonds with other polar molecules or ionized compounds.
When interactions are between two polar compounds, a dipole-dipole interaction will occur. In this type of electrostatic interaction, the partially positive end of the compound will interact with the negative end of the other compound. This specific interaction is very weak as there is no formation of permanent bonds.
In many molecules, there are permanent dipoles due to the difference in electronegativities of atoms sharing chemical bonds.
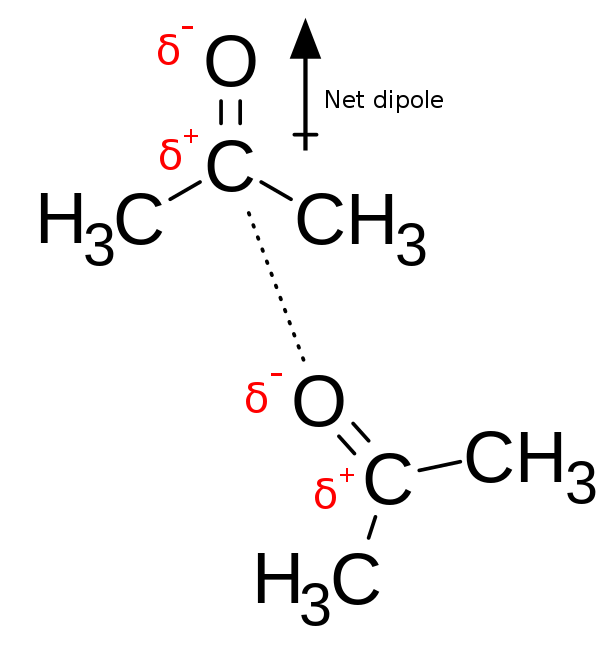
Dipole-dipole interactions between two acetone molecules, with the partially negative oxygen atom interacting with the partially positive carbon atom in the carbonyl
Image: “Chemical diagram showing dipole-dipole interactions between two molecules of acetone” by Innerstream. License: Public DomainIn ion-dipole interactions, an ion close to a polar molecule will be attracted to the end of the polar molecule which has a partial opposite charge to it. When there is an appropriate distance between the two molecules, an electrostatic interaction will happen. This will mean a positive ion will go near the negative end of the polar molecule, while a negative ion will approach the polar molecule in its positive end. Many drug molecules have ionized functional groups. The charges on these groups will bind BIND Hyperbilirubinemia of the Newborn with permanent dipoles.
This type of bonding plays a key role in the water solubility of a drug.
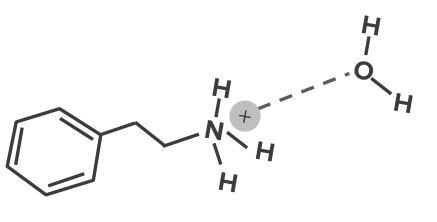
Ion-dipole bond
Image by Lecturio.This type of complex is formed between electron donor groups adjacent to electron acceptor groups. The relative positive or negative molecules transfer portions of its charges to the other molecule. In the process, a weak electrostatic bond is formed.
This is a very weak form of bonding which only occurs when the non-polar sections of molecules aggregate together in the presence of water because of the low water solubility. Hydrophobic molecules don’t like to interact with polar water molecules, and so when water is present, their tendency is to aggregate and be closer to each other.
Van der Waals bonds exist between all atoms. They arise because the electron cloud associated with an atom or molecule is constantly moving so that the electrons are never evenly distributed. Small, local, instantaneous dipoles (charge separations) occur. Diploes behave like small magnets and will attract one another.
Van der Waals forces Van der Waals forces DNA Types and Structure are weak. The larger the surface area and the larger the number of electrons in the molecules the larger the interaction will be. These interactions will only occur between molecules very close together: 0.4—0.6 nm apart. The forces drop off quickly if the molecules move apart. Force equals 1/d6 where d is the distance between the molecules.
These forces are insignificant for individual atoms but can be important in parts of molecules with lots of atoms, especially if the surfaces of the molecules are the right shapes to allow for a close fit.
Hydrogen bonds are special types of dipole interactions. They are formed when functional groups are present which contain N, O, or S and there is H linked to one of these atoms. For example, between two molecules of water:
Hydrogen bonds are important not only in drug-target interactions but also in holding together the structure of proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis and DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure.

Hydrogen bonds
Image by Lecturio.Ionic bonds, formed between molecules with opposite charges, are strong and can act across long distances. Drugs are often ionized and the active sites in receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors contain charged groups (carboxylic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance and amines).
London dispersion Dispersion Central tendency is a measure of values in a sample that identifies the different central points in the data, often referred to colloquially as “averages.” The most common measurements of central tendency are the mean, median, and mode. Identifying the central value allows other values to be compared to it, showing the spread or cluster of the sample, which is known as the dispersion or distribution. Measures of Central Tendency and Dispersion forces may also cause interactions between a drug and a receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors. The interaction is very weak as there is no permanent dipole present in the molecules. This means the positive and negative ends only occur for a very short span of time. They usually arise because of the uneven distribution of electrons at a specific time period.
In order for drugs to fit an active site Active site Area of an enzyme that binds to specific substrate molecules in order to facilitate a reaction. Basics of Enzymes, they must first have the correct shape.
Usually, only one isomer of a drug will have the required activity. Most natural molecules have stereocentres and occur in single optical isomers (e.g., the amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids which make up proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis, and hence receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors and enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes).
However, drugs should be administered as a single enantiomer, since the mirror images may have other effects including:
These occur when there is no free rotation Rotation Motion of an object in which either one or more points on a line are fixed. It is also the motion of a particle about a fixed point. X-rays around a bond (e.g. double bonds). The functional groups end up in different places.
Molecules can adopt preferred shapes although they can exist in other conformations (e.g. cyclohexane). If the drug has a preferred conformation that fits the active site Active site Area of an enzyme that binds to specific substrate molecules in order to facilitate a reaction. Basics of Enzymes, then it will usually bind BIND Hyperbilirubinemia of the Newborn more easily than if it needed to adopt an alternative shape.
Any system will adopt the lowest energy configuration. In chemical systems, this will mean that the participant will form as many bonds as possible of the strongest type. For example, water molecules will create H-bonds with each other. In ice, each molecule makes 4 H-bonds. In liquid water, H-bonds are continuously forming and breaking—on average each molecule makes 3.4 H-bonds. At any one time, liquid water can have highly H-bonded clusters and areas with few H-bonds.
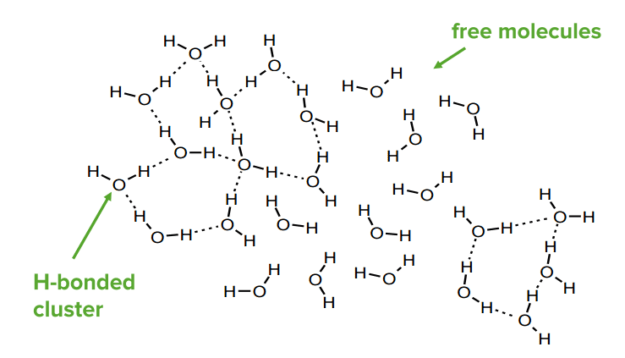
Hydrogen bonds in water
Image by Lecturio.