Carbohydrates are one of the 3 macronutrients, along with fats Fats The glyceryl esters of a fatty acid, or of a mixture of fatty acids. They are generally odorless, colorless, and tasteless if pure, but they may be flavored according to origin. Fats are insoluble in water, soluble in most organic solvents. They occur in animal and vegetable tissue and are generally obtained by boiling or by extraction under pressure. They are important in the diet (dietary fats) as a source of energy. Energy Homeostasis and proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis, serving as a source of energy to the body. These biomolecules store energy in the form of glycogen and starch, and play a role in defining the cellular structure (e.g., cellulose). Carbohydrates can be broadly classified into simple and complex carbohydrates based on the number of sugar units and chemical bonds. Carbohydrates are the most abundant organic molecules in nature and are the structural basis of many organisms. These biomolecules have a carbon:water ratio of 1:1 and a general molecular formula that can be represented as Cn(H2O)n.
Last updated: Dec 15, 2025
Carbohydrates are composed of carbon, hydrogen, and oxygen; they are defined as aldehydes or ketones of polyalcohols and have a general molecular formula of Cn(H2O)n. Carbohydrates are named based on the number of polymers (number of units) present in the molecule:
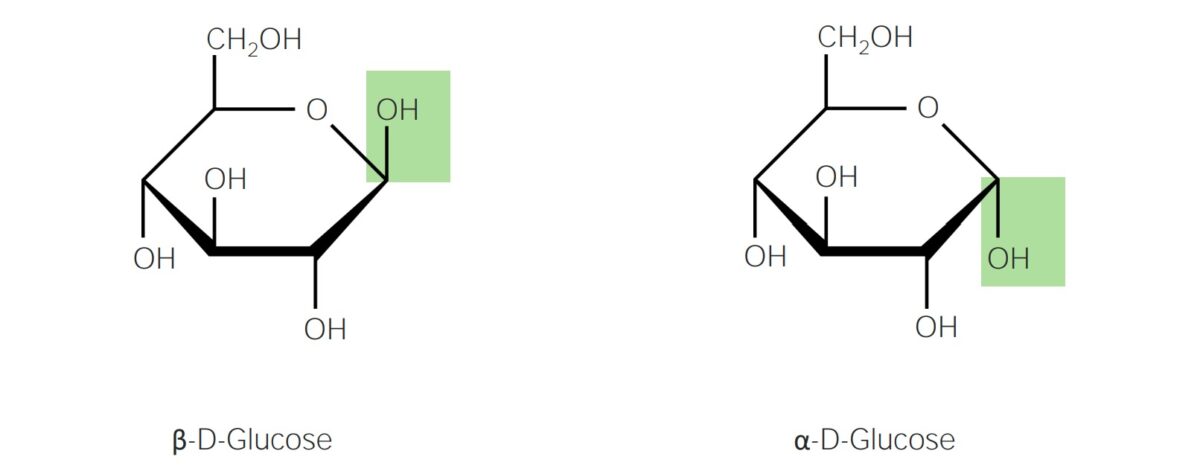
Haworth projection (glucose being the reference molecule)
Image by Lecturio. License: CC BY-NC-SA 4.0Carbohydrates are classified into simple and complex carbohydrates based on their polymerization.
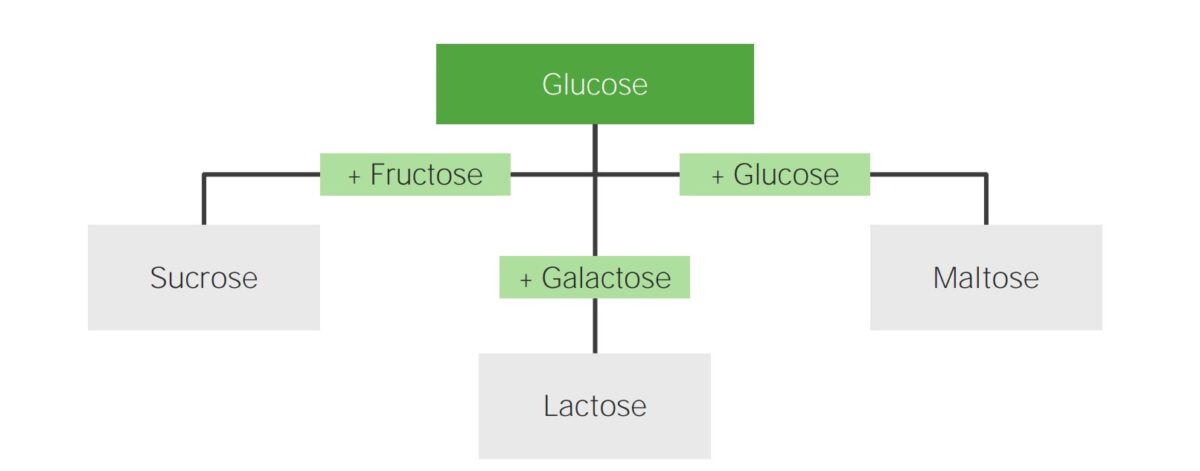
Disaccharides:
Sucrose, lactose, and maltose are common disaccharides. The image highlights the composition of these carbohydrates.
| Type of carbohydrate | Examples |
|---|---|
| Monosaccharides |
|
| Disaccharides |
|
| Oligosaccharides |
|
| Homopolysaccharides |
|
| Heteropolysaccharides |
|
| Mucopolysaccharides (GAGs) |
|
| Glycoproteins |
|
| Proteoglycans (mucoproteins) |
|
Stereoisomerism:
Compounds having the same structural formula but differing in spatial configuration are called stereoisomers:
Enantiomers: refers to two stereoisomers that are mirror images of one another
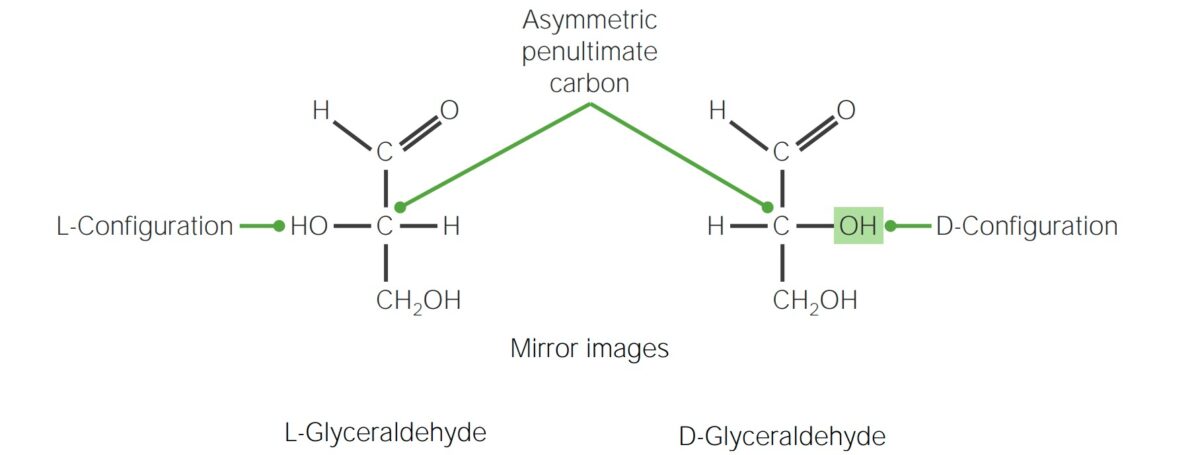
Stereoisomers with glyceraldehyde as the reference molecule
Image by Lecturio. License: CC BY-NC-SA 4.0Diastereoisomers:
Diastereoisomers are nonsuperimposable stereoisomers that are not mirror images of each other.
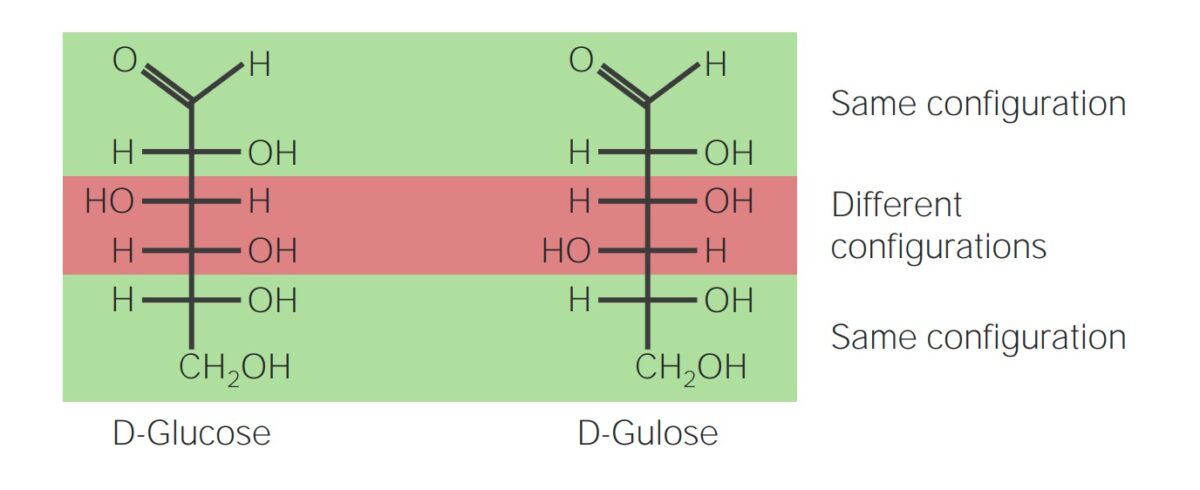
Diastereoisomer:
Glucose and gulose are both 6-carbon monosaccharides (known as hexoses) and diastereoisomers. Although they have the same chemical structure, the spatial orientation of the OH and H groups around the 2 middle, asymmetric carbons are different, which gives the molecules slightly different properties.
Epimers:
Epimers are compounds that differ from each other only in configuration with regard to a single carbon atom, other than the reference carbon atom. Glucose Glucose A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Lactose Intolerance and galactose Galactose An aldohexose that occurs naturally in the d-form in lactose, cerebrosides, gangliosides, and mucoproteins. Deficiency of galactosyl-1-phosphate uridyltransferase causes an error in galactose metabolism called galactosemia, resulting in elevations of galactose in the blood. Lactose Intolerance are epimers.
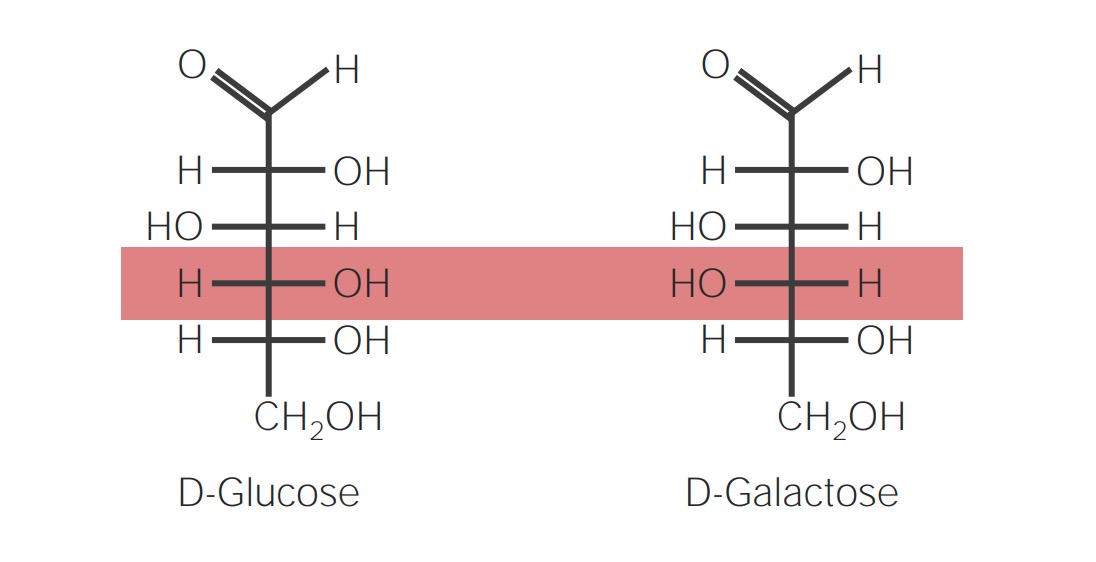
Epimer:
Image demonstrating epimers: compounds that differ in configuration around only 1 stereocenter
Anomerism:
Anomerism refers to different spatial configurations around the anomeric carbons.
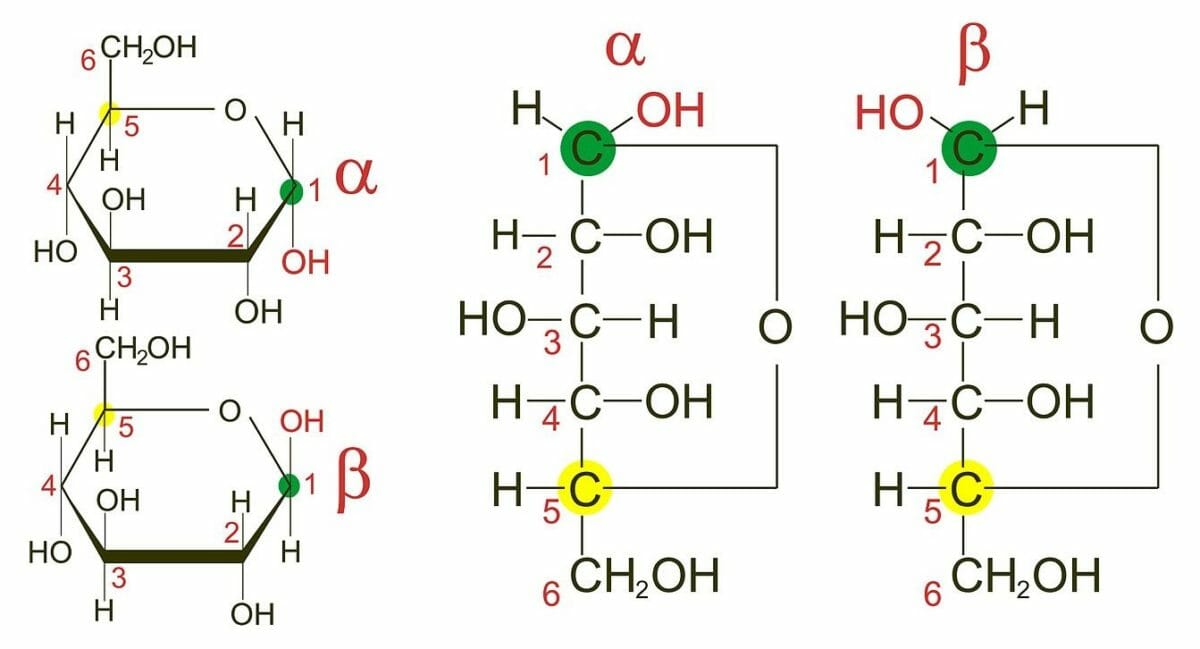
α and β anomers of D-glucose
Image: “Glucose anomers in Haworth and Fischer projections” by miguelferig. License: Public DomainOptical activity:
Optical activity is the ability of stereoisomers to rotate plane-polarized light. The molecules are designated dextrorotatory (+) or levorotatory (-) depending on the direction of light rotation Rotation Motion of an object in which either one or more points on a line are fixed. It is also the motion of a particle about a fixed point. X-rays.
Glycosidic bonds:
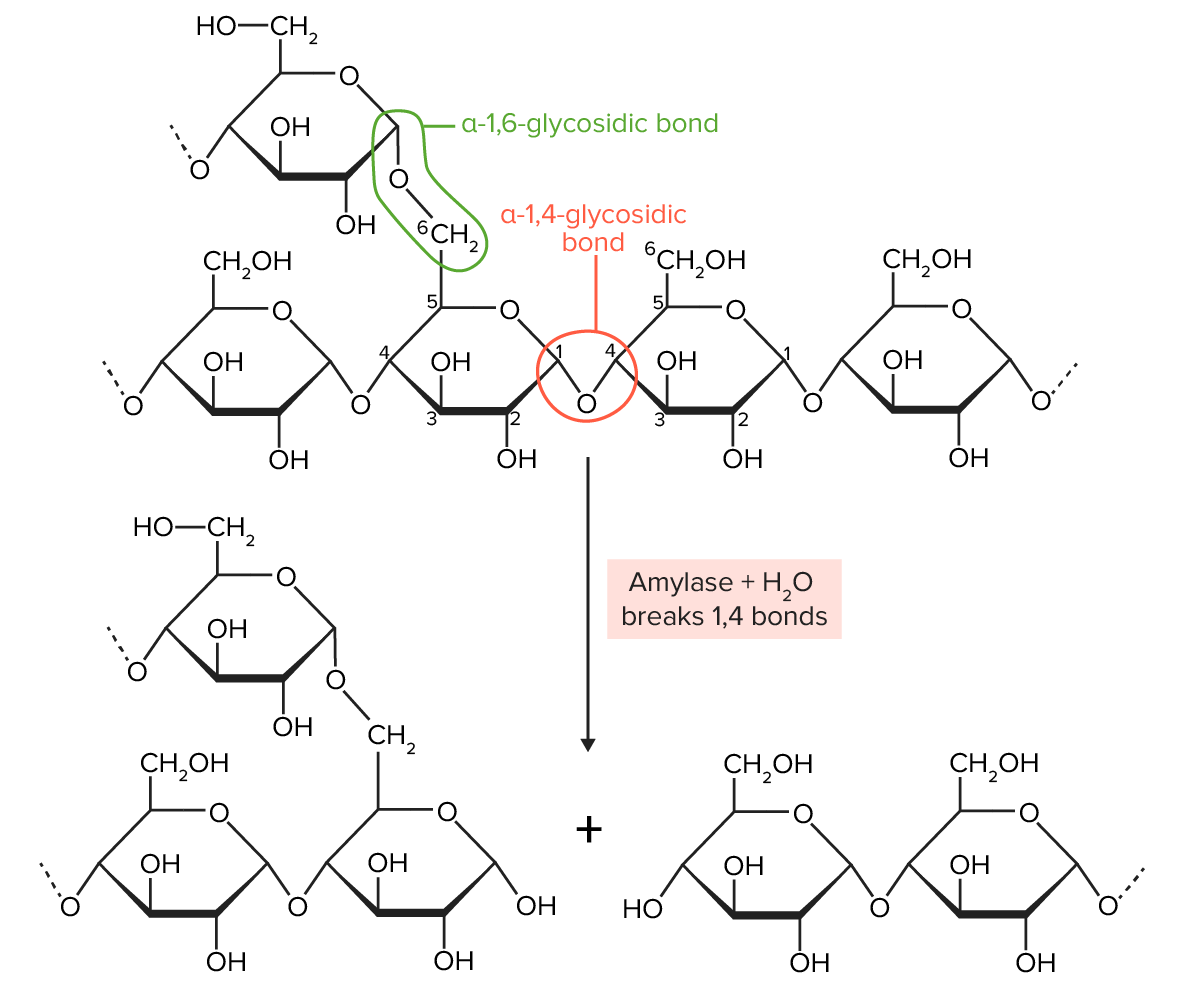
Amylopectin structure:
Amylopectin molecules are chains of glucose joined together by α-1,4-glycosidic bonds (which create a straight chain of glucose molecules) and α-1,6-glycosidic bonds (which create a branch off of the straight chain). Amylase breaks the α-1,4-glycosidic bonds, but not the α-1,6-glycosidic bonds.
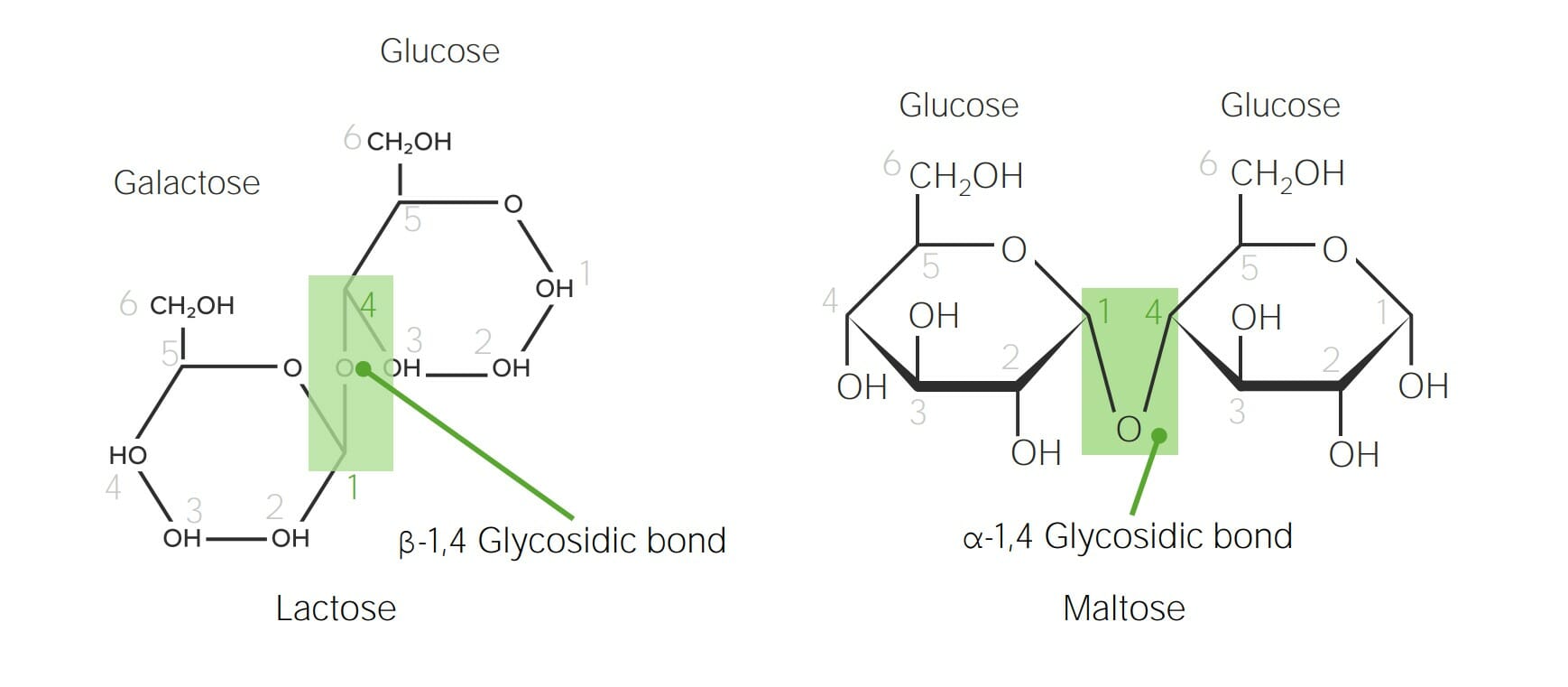
Chemical structure of lactose and maltose demonstrating α versus β glycosidic bonds:
The anomeric carbon in galactose (C1) is in the β configuration (hydroxyl group is pointing up); therefore, when galactose bonds with the C4 in glucose, a β-1,4-glycosidic bond is formed. Maltose is made up of 2 glucose molecules. The anomeric carbon in glucose (C1) is in the α configuration (hydroxyl group is pointing down); therefore, the bond in maltose is an α-1,4-glycosidic bond between 2 glucose molecules.
Oxidation and reduction reactions:
Carbohydrates play many roles in the sustenance of life. Primarily, carbohydrates serve as a source of nutrition. Additionally, they are structural components of cells, and partake in immune defense and intracellular communication Communication The exchange or transmission of ideas, attitudes, or beliefs between individuals or groups. Decision-making Capacity and Legal Competence.
Carbohydrates are central to many metabolic pathways in the body, which include the formation, breakdown, and interconversion of carbohydrates.
Key metabolic pathways:
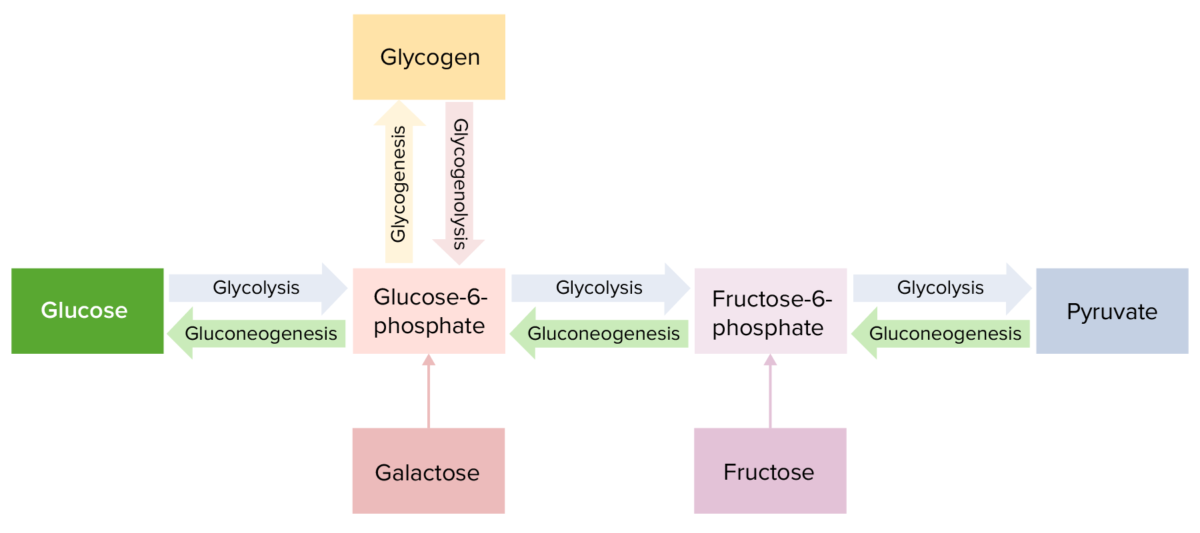
Diagram highlighting the pathways of carbohydrate metabolism
Image by Lecturio. License: CC BY-NC-SA 4.0Several conditions are associated with disorders in carbohydrate metabolism: