Hydrocarbons are compounds exclusively composed of carbon and hydrogen atoms and can be considered aliphatic or aromatic; aromatic compounds will be the primary focus Focus Area of enhancement measuring < 5 mm in diameter Imaging of the Breast within this article. In general, aromatic compounds exhibit aromaticity, the property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit increased stabilization compared to ordinary conjugation Conjugation A parasexual process in bacteria; algae; fungi; and ciliate eukaryota for achieving exchange of chromosome material during fusion of two cells. In bacteria, this is a unidirectional transfer of genetic material; in protozoa it is a bi-directional exchange. In algae and fungi, it is a form of sexual reproduction, with the union of male and female gametes. Bacteriology. In this article, the different properties of an aromatic compound are discussed as well as their different applications; included is a discussion on benzene, C6H5. Unlike ordinary alkenes where addition reaction occurs, aromatic compounds involve electrophilic aromatic substitution ( EAS EAS Rectum and Anal Canal: Anatomy).
Last updated: Mar 7, 2022
Historically, aromatic compounds referred to any compound producing a fragrant smell Smell The sense of smell, or olfaction, begins in a small area on the roof of the nasal cavity, which is covered in specialized mucosa. From there, the olfactory nerve transmits the sensory perception of smell via the olfactory pathway. This pathway is composed of the olfactory cells and bulb, the tractus and striae olfactoriae, and the primary olfactory cortex and amygdala. Olfaction: Anatomy, such as benzaldehydes which produce the scent of cherries, peaches, and almonds. However, some aromatic compounds were later discovered that do not exhibit this property. Therefore, aromatic compounds are currently classified according to their chemical behavior.
Aromatic compounds are a large family of compounds comprised of a six-membered ring or more complex structure. Notably, aromatic compounds are not limited to hydrocarbons as there are aromatic compounds like thiophene and pyridine which have S and N in their structure. Ions can also be considered aromatic as long as they exhibit the properties noted above.
Compounds are classified as aromatic if they are able to exhibit the following properties:
The compound is considered to be aromatic if it is able to exhibit all of the properties included in the list. Otherwise, the compound can be classified as either a non-aromatic or anti-aromatic compound.
An aromatic compound is considered a big family of compounds as it comprises of compounds with a six-membered ring, to more complex structures. Aromatic compounds are also not limited to hydrocarbons as there are also aromatic compounds like thiophene and pyridine that are considered aromatic, even if they have S and N in their structure. Ions can also be considered as aromatic, as long as they will exhibit the properties included above.
Steroids Steroids A group of polycyclic compounds closely related biochemically to terpenes. They include cholesterol, numerous hormones, precursors of certain vitamins, bile acids, alcohols (sterols), and certain natural drugs and poisons. Steroids have a common nucleus, a fused, reduced 17-carbon atom ring system, cyclopentanoperhydrophenanthrene. Most steroids also have two methyl groups and an aliphatic side-chain attached to the nucleus. Benign Liver Tumors are aromatic compounds which are important to biological organisms and include hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types responsible for the growth and the development of physical features. In the pharmaceutical industry, compounds such as atorvastatin Atorvastatin A pyrrole and heptanoic acid derivative, hydroxymethylglutaryl-CoA reductase inhibitor (statin), and anticholesteremic agent that is used to reduce serum levels of ldl-cholesterol; apolipoprotein b; and triglycerides. It is used to increase serum levels of hdl-cholesterol in the treatment of hyperlipidemias, and for the prevention of cardiovascular diseases in patients with multiple risk factors. Statins, a cholesterol-lowering drug, are also aromatic. Pyridine, C5H5N, is a common aromatic compound present in vitamins and other pharmaceuticals. Most of the essential vitamins we need are also aromatic. Benzene, the most common aromatic compound, is used in manufacturing polystyrene plastics, pesticides, and some pharmaceutical drugs.
Benzene is a hydrocarbon with the molecular formula C6H6 and has a cyclic structure with alternating or conjugated double bonds Conjugated Double Bonds Polyenes. Although benzene is unsaturated, it does not exhibit the relative reactivity of its other alkene and acyclic counterparts. For example, cyclohexene readily reacts with Br2 to produce 1,2-dibromocyclohexane. Conversely, benzene reacts very slowly with Br2 to form bromobenzene which still retains the presence of three double bonds, demonstrating the relative stability of benzene.
Another important observation is the bond lengths in a benzene molecule. In ordinary compounds, single bonds are expected to be longer than double bonds. However, the bond length analysis of benzene shows that the C to C bond lengths are all equal, regardless of being a single or double bond. The bond angle in each C-C-C segment is 120°, forming a perfect hexagon shape. The electrostatic potential map shows that the electron density is identical in all six carbon-carbon bonds. Each carbon also has a p-orbital that lies perpendicular to the plane of the six-membered ring.
Because of the peculiar properties of benzene, each double bond is not considered to be localized, but rather, overlapping to only one other p-orbital. These properties are only possible if the compound has all p-orbitals equally overlapping adjacent p-orbitals. This overlap of orbitals enables delocalization of the 6 π electrons around the ring. Because of this, it is difficult to isolate the 2 possible isomers of benzene. Instead, a circle is included inside the 6 sides of the molecule to indicate delocalization of the π electrons.
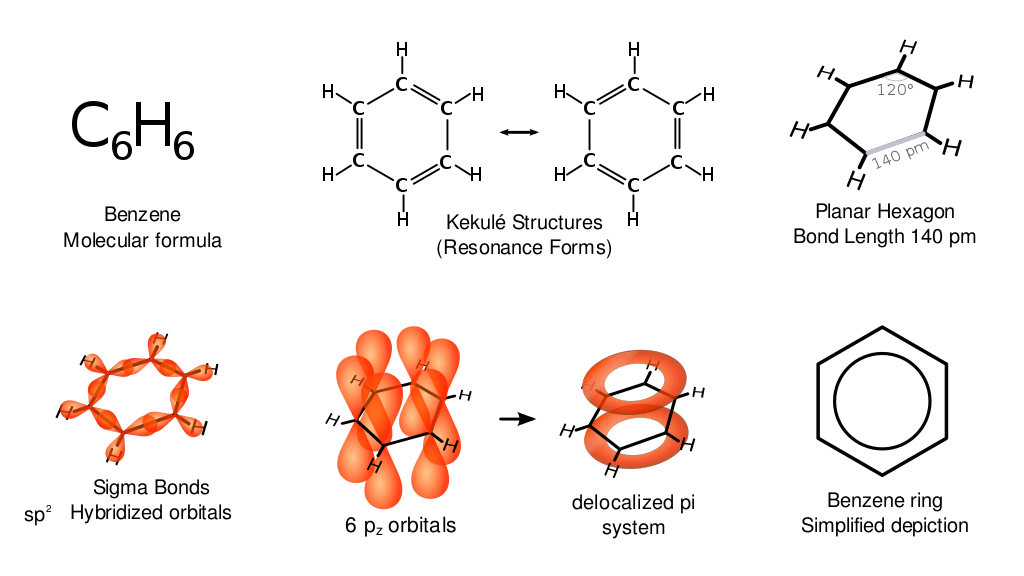
Various representations of benzene
Image: “Various representations of Benzene” by Nneonneo. License: CC BY-SA 3.0Electrophilic aromatic substitution ( EAS EAS Rectum and Anal Canal: Anatomy) occurs when an electrophile reacts with an aromatic ring, substituting one of the H atoms in the ring. A number of different electrophiles may be used in EAS EAS Rectum and Anal Canal: Anatomy. Possible electrophiles include halogens (-Cl, -Br, -I), the sulfonic acid group (-SO3H), hydroxyl group (-OH), nitro group Nitro Group Nitroimidazoles (-NO2), acyl groups (-COR), and alkyl groups (-R). Even with benzene alone, one can come up with some compounds because of the number of possible electrophiles for the reaction.
Aromatic compounds, unlike ordinary alkenes, are less reactive compared to their acyclic counterparts due to their relative stability afforded by π electron delocalization. In a halogenation reaction known as bromination, Br2 can readily react with ethane to produce dibromoethane. This is not true for benzene. For the bromination reaction to proceed in benzene, a catalyst such as FeBr3 is needed which provides a different mechanism for the reaction to proceed.
The 1st step in the reaction is the polarization of the Br2 molecule by FeBr3. In the process, a more electrophilic molecule is produced in the form of FeBr4– Br+. The presence of Br+ makes FeBr4– Br+ more electrophilic than an ordinary Br2 molecule. The FeBr4– Br+ will then attack the benzene molecule and a base will remove the proton in the process. Below is the mechanism of the reaction.
Nitration of aromatic rings can be achieved using a mixture of concentrated nitric and sulfuric acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance. The electrophile in the reaction is the nitronium ion, NO2+, which is produced by the protonation and loss of water in the form of HNO3. Just as in halogenation, a carbocation intermediate is produced when the nitronium ion interacts with the benzene ring. Upon loss of a proton, the neutral substitution product Product A molecule created by the enzymatic reaction. Basics of Enzymes nitrobenzene is produced. Below is the basic mechanism for the nitration of benzene.
A sulfonation reaction of aromatic rings is achieved by reacting benzenes with fuming sulfuric acid Sulfuric acid Inorganic and organic derivatives of sulfuric acid (h2so4). The salts and esters of sulfuric acid are known as sulfates and sulfuric acid esters respectively. Caustic Ingestion (Cleaning Products) (a mixture of H2SO4 and SO3). The electrophile for the reaction is either HSO3+ or neutral SO3, depending on the reaction conditions. This reaction mechanism is similar to the bromination and nitration reactions previously discussed. A sulfonation reaction is favored in the presence of strong acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, while desulfonation is favored in hot, dilute aqueous acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance. The mechanism for sulfonation is as follows:
A hydroxylation reaction of aromatic rings is very difficult to achieve in ordinary reaction conditions and requires the presence of biological enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes. An example of a hydroxylation reaction catalyzed through a biological pathway is the hydroxylation of p-hydroxyphenylacetate using the enzyme p-hydroxyphenylacetate-3-hydroxylase to produce 3,4-dihydroxyphenylacetate. The process requires molecular oxygen plus the coenzyme-reduced flavin adenine Adenine A purine base and a fundamental unit of adenine nucleotides. Nucleic Acids dinucleotide, FADH2.
An alkylation reaction proceeds when an alkylchloride is combined with AlCl3. AlCl3 enables the dissociation Dissociation Defense Mechanisms of R-X to produce the carbocation that will serve as the electrophile. The electrophile then attacks the benzene ring and the reaction is completed by proton loss. The mechanism of the reaction is shown below.
The mechanism of the acylation reaction is similar to the alkylation reaction. The alkyl halide is just replaced by an acyl halide. For example, for the acylation reaction of benzene, the first step is the generation of a strong electrophile by the interaction between the acyl chloride Chloride Inorganic compounds derived from hydrochloric acid that contain the Cl- ion. Electrolytes and the AlCl3 molecule. The acyl cation is then stabilized by rearrangement in the acyl cation with the + charge present in the C atom. The +C then interacts with the double bond in the benzene molecule, forming a carbocation that can be stabilized by abstracting one of the protons using the AlCl4– ion produced in the first step. The mechanism for acylation is shown in the figure below.