Understanding acids and bases can be a challenge. It gets even more complicated when you have to understand how they’re balanced in the body. However, diagnosing and treating an acid-base disorder can save lives. In this article, I’ll show you that identifying them can be as easy as A-B-G.
What Is Arterial Blood Gas?
An ABG test checks the acid-base balance in your blood as well as the partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2). The components include:
- pH: This is the measure of acids and bases in the blood. Basically, if it’s lower than normal, it’s acidic. If it’s higher, it’s basic.
- PaO2: This is the pressure of oxygen absorbed into the blood. This value shows how efficiently oxygen moves from the lungs to the blood.
- PaCO2: This is the measure of CO2 in the blood and how well it moves.
- HCO3: This is the basic compound made from carbon dioxide. The higher this value, the more basic the blood.
- SaO2: This is how much oxygen-carrying hemoglobin is in your blood.
The ABG test is one of the most common tests performed on patients in intensive care units. Although you can measure things like oxygen saturation through an oximeter, it’s still pretty standard to order an ABG to get a better understanding of the acid-base balances in a patient. Remember that any imbalance can make critically ill patients unstable.
How to Get an ABG
In different hospitals, the ABG samples are taken by different people. Some places have respiratory therapists who take the samples. Other places have the physicians or nurses collect them. Where I did my internship, it was the interns who took it because if you order something, you should at least know how it’s done yourself. So, just in case you’re asked to collect ABG as a student, here’s how to do it:
The first task is performing the Allen test. This is to make sure that there’s still collateral blood flow to the hand after an arterial puncture, which can disrupt the blood flow in the affected artery. Usually, the sample is taken from the radial artery. How is the Allen test done? First, you occlude both the radial and ulnar artery with the patient’s fist clenched. After 5 seconds, ask the patient to unclench their fist and observe the color of the hand. If the arteries are occluded correctly, the palm should be pale. Next, release the ulnar artery. The color of the hand should be back to normal within 10-15 seconds. Otherwise, you’ll need to find another point of extraction like the femoral or brachial artery.
Once it’s assured that there will still be collateral blood flow to the hands, palpate for the radial artery. You’ll know it’s the artery because you will feel strong pulsations. As you clean and disinfect the area, you can use this time to explain to the patient what you’re about to do.
Be aware that this procedure is painful. The radial artery is deep and surrounded by nerves. As such, it’s best that you inform the patient beforehand.
The needle is then inserted with a special syringe containing an anticoagulant, to stop the blood from clotting. After that, the specimen is sent to the lab within 30 minutes.
Why Is Getting an ABG Important?
Derangements in acid-base balance can be the result of respiratory, circulatory, and metabolic problems. This can destabilize the patient and, if they’re already in critical condition, they’ll deteriorate quickly. Many diseases can be evaluated with this test, but are not limited to:
- Sepsis
- Acute Respiratory Distress Syndrome (ARDS)
- Diabetic Ketoacidosis
- Cardiac Arrest
- Asthma in Acute Exacerbation
As you can see, a lot of these diseases are emergent cases. Of course, while the main goal is first to stabilize the patient, the patient can destabilize again if you don’t treat the underlying condition. So, it’s important that when the time comes, you know how to interpret the patient’s lab values quickly, including the ABG. Similar to that of electrolytes, imbalances in acid-base can be life-threatening. Normally, we have our lungs and kidneys working together to maintain that balance. So, when the proper function of either of these organs is disturbed, you’ll have an abnormal ABG.
How to Interpret ABG Values
Interpreting ABG values can be difficult. In medical school, I always got the steps confused and I was really slow at reading them. I also got confused as to which values determined acidosis or alkalosis, and I was completely thrown off when I realized that mixed disorders exist. Below, I’ll break down the steps so that they’re easier to follow:
Get a patient history
In medical school, you’ll usually just be given values. However, in real life, you can always evaluate the patient’s case and figure out the most likely diagnosis. For example, if the patient has uncontrolled diabetes and is hypotensive, you might consider metabolic acidosis. If the patient has COPD or muscular weakness, you might think respiratory acidosis. Keep the patient’s history in mind as you read through their ABG values. Abnormal values happen for a reason in context with the condition of the patient.
What are normal ABG values?
Memorize the normal values of an ABG test. I don’t have a fancy mnemonic for this or an easy way to memorize it. I just remember them through a lot of practice and repetition. Here are the normal values, but take note that these ranges may vary for different age groups:
- pH: 7.35–7.45
- PaO2: 75–100 mm Hg
- PaCO2: 35–45 mm Hg
- HCO3: 22–26 meq/L
- SaO2: 95%–100%
Identify the dominant disorder
First, look at the pH. While 7.35–7.45 is normal, any number away from 7.40 points to acidosis or alkalosis. Higher is alkalosis, lower is acidosis. It’s as simple as that.
Next, look at the paCO2, a respiratory acid. Similar to pH, while 35–45 mm Hg is the normal range, any number away from 40 means there’s acidosis (higher) or alkalosis (lower). If the derangement in paCO2 explains the change in pH, then it’s a respiratory disorder. If not, look at the HCO3.
For HCO3, the same as with pH and paCO2, any number away from 24 means that there’s acidosis (lower) or alkalosis (higher). If the derangement here explains the change in pH, then it’s a metabolic disorder.
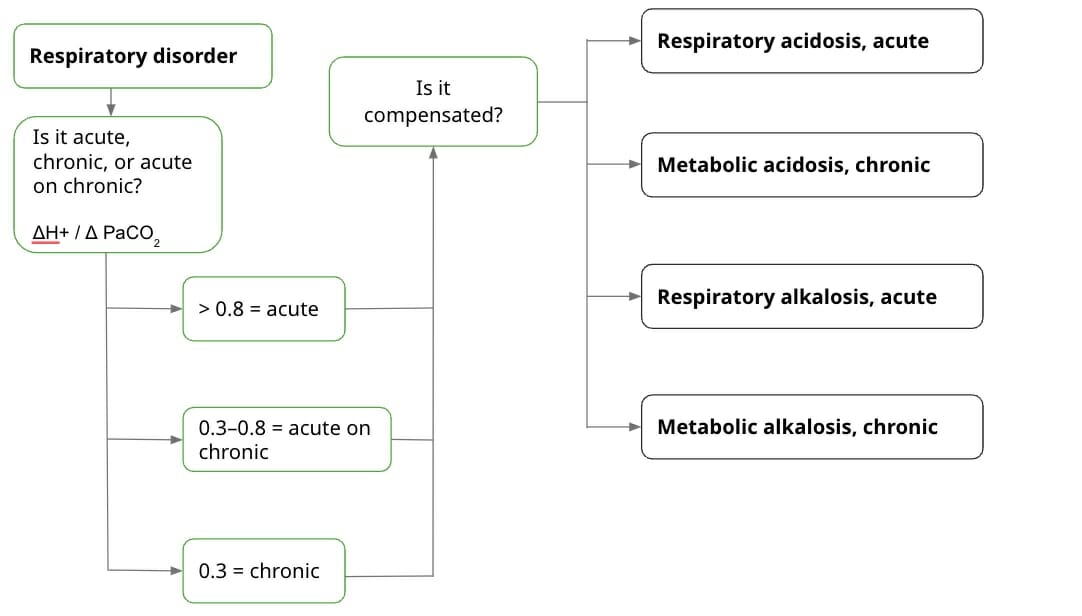
If it’s a respiratory disorder:
First, find out whether the disorder is acute, acute on chronic, or chronic. Depending on the result, you can have a better idea of the cause of the acid-base imbalance. You use the change in H+ over the change in PaCO2. The value of H+ itself corresponds to a specific pH. The “change” in H+ (ΔH+) is the H+ value of the current pH subtracted from the H+ value of normal pH. Basically, it’s this formula:
ΔH+ / Δ PaCO2
- If the value is > 0.8, it’s acute.
- If the value is 0.3–0.8, it’s acute on chronic.
- If the value is 0.3, it’s chronic.
Next, calculate whether it’s compensated. Compensating means that the body is trying to balance out the acid-base abnormality. If it’s uncompensated, it may be the failure of the lungs or kidneys. It can also be that the imbalance is so severe, the body can’t balance it out anymore. Of course, no one wants either of these to happen, so it’s good to know whether the body is still able to balance itself out.
There are a lot of formulas out there for compensation, and it’s good that you learn them. But I missed the opportunity to understand where the formulas come from because I was just fed them. Instead, I’m going to list the rules for compensation. Basically for every increase/decrease in paCO2 by 10 mm Hg, there should be a compensatory increase/decrease in HCO3. So, if one value goes up, the other also goes up, and vice versa.
- Respiratory acidosis, acute: [HCO3-] increase by 1 mEq/L for every 10 mm Hg increase in paCO2 above 40
- Respiratory acidosis, chronic: [HCO3-] increase by 3.5 mEq/L for every 10 mm Hg increase in paCO2 above 40
- Respiratory alkalosis, acute: [HCO3-] decrease by 2 mEq/L for every 10 mm Hg decrease in paCO2 below 40
- Respiratory alkalosis, chronic: [HCO3-] decrease by 5 mEq/L for every 10 mm Hg decrease in paCO2 below 40
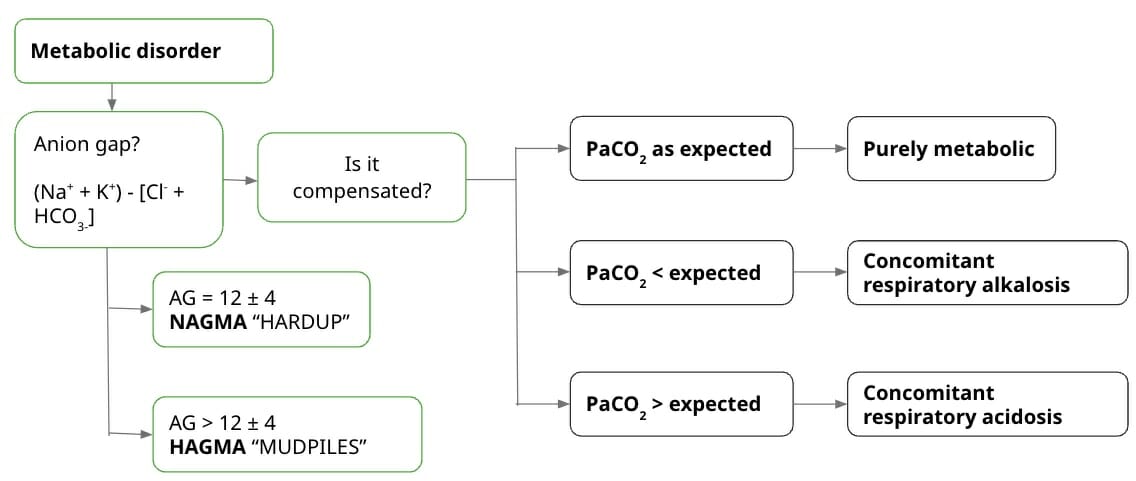
If it’s a metabolic disorder:
Usually what’s asked of students is the anion gap (AG), which is affected by the amount of anions and cations. Since electroneutrality should be maintained, the “gap” in the anion gap refers to mostly weak acids like albumin. When the anion gap is high, this means that the gap is higher than what albumin produces. This points to other anions, such as lactates and ketones. Basically, this is the formula:
AG = (Na+ + K+) – [Cl- + HCO3-]
Sometimes K+ is omitted from the equation because it’s usually found in low concentrations. Using K+ also changes the normal range for the anion gap. So, if you include K+ in the computation, the normal range is 12 ± 4. If you exclude it, the normal range becomes 8 ± 4. High values indicate High Anion Gap Metabolic Acidosis, or HAGMA. Meanwhile, normal values indicate Normal Anion Gap Metabolic Acidosis, or NAGMA. This is useful to know because it can narrow down the causes of acidosis in the patient.
There are multiple mnemonics for the causes of metabolic acidosis, but the most commonly-used is the one for HAGMA: MUDPILES. This stands for:
- Methanol
- Uremia
- Diabetic ketoacidosis
- Propylene glycol
- Isoniazid/Iron
- Lactic acidosis
- Ethylene glycol
- Salicylates
For NAGMA, I usually use HARDUP. This one stands for:
Hyperalimentation
- Acetazolamide
- Renal tubular acidosis
- Diarrhea
- Ureterosigmoid fistula
- Pancreaticoduodenal fistula
Next, find out whether the derangement is purely metabolic or whether there’s a concomitant respiratory disorder to compensate for the metabolic disorder. To do this, you compute the expected PaCO2. Basically, for every mEq/L point increase/decrease in HCO3 from 21, there is a compensatory increase/decrease in PaCO2 by 0.7 mm Hg. It follows this formula:
Expected PaCO2 = [0.7 × HCO3-+ 21] ± 2
If the actual PaCO2 falls within normal range, it’s a purely metabolic disorder. If the actual is lesser than the expected, there’s respiratory alkalosis. If the actual is greater than the expected, there’s respiratory acidosis.
In Summary
That’s a lot of information to take in. So, let’s try to sum up the steps you’ve read:
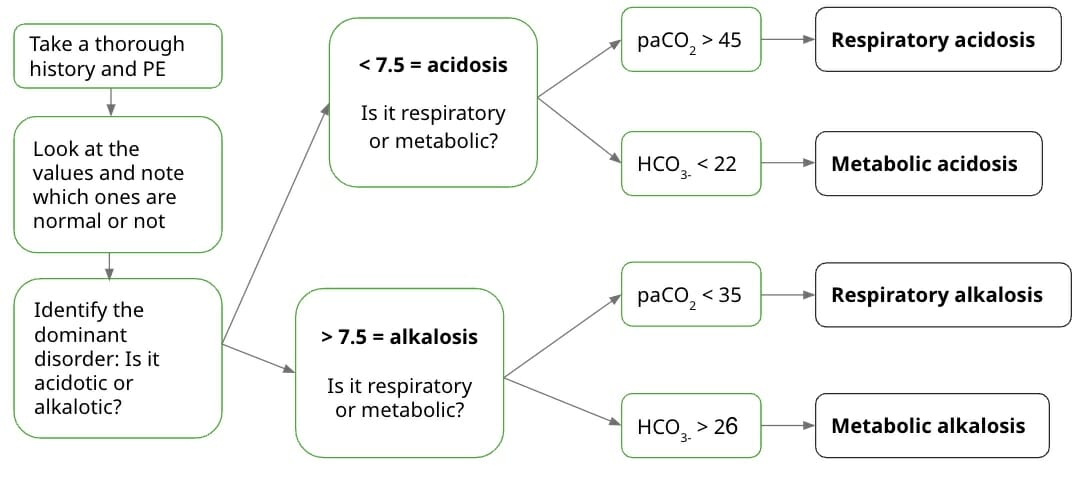
This is a summary of the first few steps before moving on to the next, depending on the dominant disorder. Take note that you should always look at the PaCO2 levels first and see whether they match the pH derangement, before looking at the HCO3 values. Next, students are usually asked whether the values are compensated or uncompensated, and for other details depending on the dominant disorder.
ROME method
To make things simpler, I’ll explain the ROME Method, which I find to be the quickest way to read ABG results, especially when you’re pressed for time. There’s also the Tic-Tac-Toe Method, but I find the ROME Method a lot faster because I don’t have to draw anything. Usually, you’ll be asked 3 things: the acid-base imbalance, whether it’s respiratory or metabolic, and whether it’s compensated, partially compensated, or uncompensated.
First, look at the pH and determine whether the disorder is acidosis or alkalosis. Next, figure out the dominant disorder.
ROME stands for “Respiratory, Opposite; Metabolic, Equal”.
What does that mean? If the CO2 and the pH are opposite each other (one value is increased while the other is decreased), then the disorder is respiratory. If the HCO3 and the pH are equal (both values are increased or decreased), then the disorder is metabolic. It’s as simple as that.
Finally, determine the compensation. Remember that when CO2 increases or decreases, HCO3 should compensate (and vice versa), to the point that it goes over the normal range. For example, when CO2 increases during respiratory acidosis, HCO3 should also increase. If that happens, and the pH is within the normal range of 7.35–7.45, then it is fully compensated. The body is compensating — and succeeding. If there is compensation, but the pH is still abnormal, then it is only partially compensated. The body is compensating, but it’s not quite there yet. Finally, if the other value is within normal range, that means it is uncompensated because the body is not even trying.
Take the Course: Physiology
Cover all physiology essentials with Dr. Thad Wilson, Kentucky University